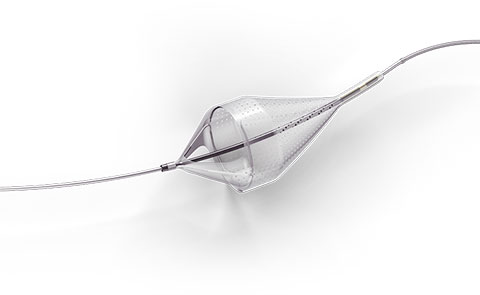
Capture with Confidence
Indicated for Carotids and Lower Extremities
The Emboshield NAV6™ Embolic Protection System, which includes BareWire™ Filter Delivery Wires, allows the guide wire to rotate and advance freely, independent of the Emboshield NAV6™ filter.*
The Emboshield NAV6™ EPS is indicated for use as a guide wire and embolic protection system to contain and remove embolic material (thrombus / debris) while performing angioplasty and stenting procedures in carotid arteries and while performing atherectomy, during standalone procedures or together with PTA and/or stenting, in lower extremity arteries. The diameter of the artery at the site of the Filtration Element placement should be between 2.5 and 7.0 mm.
Embolic Protection Device for both Lower Extremity and Carotid Arteries
Captures Effectively to Minimize Downstream Complications1-3
- Centered wire design prevents bias against the vessel wall for effective debris capture.*
- Circumferential nitinol frame maintains optimal wall apposition, even on a bend.*
- Platinum-tungsten frame coils provide excellent visibility.*
- Two sizes allow for easy selection and minimal lab inventory.*
Limits Filter Movement and Maintains Wire Access Through Innovative BareWire™ Design*
- The unique BareWire™ technology allows the wire to rotate and advance freely, independently of the filter.
- The filter is designed to stay in place during device delivery.
- Continued wire access, after filter is fully retracted, allows for easy delivery of additional therapy.
Navigates Skillfully Through Carotid and Lower Extremity Vasculature*
- Various types of BareWires™—distal access, workhorse, and support—are designed for various carotid anatomies.
- They promote navigational success through torturous anatomy and challenging arches.
Is Compatible with Various Atherectomy Options4
- Emboshield NAV6™ EPS and BareWire™ are compatible with a variety of atherectomy types.
- BareWire™ is available in 190 cm and 315 cm lengths.
In carotid procedures, the Emboshield NAV6™ EPS is used during stenting with the RX Acculink™ Carotid Stent System and the Xact™ Carotid Stent System.
* Data on file at Abbott.
References
- Bioangiu et al. Comparative analysis of retrieved particulate debris after superficial femoral atherectomy using three different atherectomy methods. EuroIntervention, May 2012.
- Bioangiu et al. Analysis of Retrieved Particulate Debris after Superficial Femoral Artery (SFA) Atherectomy Using the Pathway Jetstream G3 device. CCI, May 2011, 77(2) p. S57.
- Mendes et al. Clinical significance of embolic events in patients undergoing endovascular femoropopliteal interventions with or without embolic protection devices. JVS, February 2014, 59(2), 359-367.
- Philips Turbo-Elite, Medtronic TurboHawk PPES, and Boston Scientific Jetstream XC and SC. Data on file at Abbott.
MAT-2114539 v2.0
Emboshield NAV6 ™ Embolic Protection System

Indications
The Emboshield NAV6 ™ Embolic Protection System is indicated for use as a guide wire and embolic protection system to contain and remove embolic material (thrombus / debris) while performing angioplasty and stenting procedures in carotid arteries and while performing atherectomy, during standalone procedures or together with PTA and/or stenting, in lower extremity arteries. The diameter of the artery at the site of the Filtration Element placement should be between 2.5 and 7.0 mm.
Contraindications
The Emboshield NAV6 ™ Embolic Protection System is contraindicated for use in
- Patients in whom anticoagulant and / or antiplatelet therapy is contraindicated.
- Patients with severe vascular tortuosity or anatomy that would preclude the safe introduction of the Guiding Catheter / Introducer Sheath, Embolic Protection System.
- Patients with a known allergy or hypersensitivity to device materials (Nitinol, Nickel, Titanium) or contrast medium, who cannot be adequately premedicated.
- Patients with uncorrected bleeding disorders.
- Lesions in the ostium of the common carotid artery.
- Inability to cross the lesion with the BareWire™ Filter Delivery Wire.
- Diffusely diseased vessels where there is no disease-free section in which to deploy the Filtration Element
- Insufficient straight section of vessel distal to the lesion to permit Filtration Element deployment.
Warnings
Use of the device should be restricted to physicians trained to the specifics of the device and to the Instructions for Use. Operators must be knowledgeable of the current medical literature and familiar with the principles, clinical applications, complications, side effects and hazards commonly associated with carotid and lower extremity interventional procedures.
General Warning
Refer to instructions supplied with all interventional devices to be used with the Emboshield NAV6 ™ Embolic Protection System for their intended uses, contraindications, and potential complications.
The Emboshield NAV6 ™ System is supplied sterile. Do not use if the package has been opened or is damaged. Carefully inspect the system components prior to use to verify that they have not been damaged and that the size, shape and condition are suitable for the procedure for which they are to be used. A device or access device that is kinked or damaged in any way should not be used.
Safety and effectiveness of this device as an embolic protection system has not been established in the coronary or cerebral vasculature.
The safety and efficacy of the Emboshield NAV6 ™ Embolic Protection System has not been demonstrated with carotid stent systems other than the Xact™ or Acculink™ Carotid Stent Systems.
The safety and efficacy of the Emboshield NAV6 ™ Embolic Protection System has not been demonstrated with atherectomy devices other than Turbo-Elite‡ Laser Atherectomy Catheter, Jetstream‡ Single Cutter (SC) Atherectomy Catheter, Jetstream‡ eXpandable Cutter (XC) Atherectomy Catheter and TurboHawk‡ Peripheral Plaque Excision System.
The Emboshield NAV6 ™ device can only be used with the BareWire™ Filter Delivery Wire. Use of the device with any guide wire other than the BareWire™ Filter Delivery Wire will lead to loss of the Filtration Element during the procedure or an inability to retrieve the Filtration Element.
This device is designed and intended for single use only. Do not reuse, reprocess or resterilize. Reuse, reprocessing or resterilization may compromise the structural integrity of the device and / or delivery system and / or lead to device failure, which may result in patient injury, illness or death. Reuse, reprocessing or resterilization may also create a risk of contamination of the device and / or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device and / or delivery system may lead to injury, illness or death of the patient.
Store in a cool, dark, dry area. Do not use the product after the Use by date specified on the label.
Overstretching of the artery may result in rupture and life-threatening bleeding.
Appropriate antiplatelet, anticoagulant and, if necessary, vasodilator therapy must be used during the procedure. Anticoagulant therapy sufficient to maintain an Activated Clotting Time of at least 250 seconds for the duration of the procedure is recommended.
Adequate guide catheter or sheath support is required for the introduction of the RX Delivery Catheter, RX Retrieval Catheter, and all interventional devices to be used during the procedure.
Maintain a snug seal between the device and the hemostasis valve during catheter insertion. Failure to observe this may result in air being drawn into the access device through the hemostasis valve. Device insertion should be performed slowly to minimize the risk of air entrainment. It is therefore recommended that flushing of contrast media (or other fluids) is performed before or after insertion of the catheter, but not while the catheter is within the access device.
Do not advance any component of the Emboshield NAV6 ™ Embolic Protection System against significant resistance. The cause of any resistance should be determined via fluoroscopy and remedial action taken.
Torquing the BareWire™ Filter Delivery Wire against resistance may cause BareWire™ Filter Delivery Wire damage and / or BareWire™ Filter Delivery Wire tip separation.
Perform all exchanges slowly to prevent air embolism or trauma to the artery.
The RX Delivery Catheter should not be pulled back quickly in the event that resistance is felt during catheter advancement. Rapid withdrawal of the RX Delivery Catheter in the presence of resistance may result in premature deployment of the Filtration Element.
In the event of complications, surgical intervention may be required.
A high pressure contrast injection may cause Filtration Element movement in the vessel.
Do not attempt to move the Filtration Element after deployment and prior to the start of retrieval. Do not torque the RX Delivery and RX Retrieval Catheters.
Allow and maintain adequate distance between the Emboshield NAV6 ™ Filtration Element and other interventional devices (including carotid stent systems and balloons) to avoid engagement.
When introducing the delivery system, confirm that the wire tip is free within the vessel lumen and is not directed into the vessel wall. Failure to do so may result in vessel trauma. Use the radiopaque marker on the interventional device to confirm position.
Avoid excessive movement of the filter basket during catheter device exchanges. Excessive movement of the deployed basket may cause vessel trauma or spasm.
After use, this device, its accessories and packaging should be appropriately classified for disposal (e.g., biohazard, sharps, non-hazardous waste, etc.) and carefully disposed of in compliance with facility procedures and applicable laws and regulations.
The following vessel anatomy specifications preclude the use of the stent system or appropriate positioning of the embolic protection system:
- Hemoglobin (Hgb) < 8 gm/dl (unless on dialysis), platelet count < 50,000, INR > 1.5 (irreversible), or heparin-associated thrombocytopenia.
- Evidence of a stroke within the previous 30 days.
- History of ipsilateral stroke with fluctuating neurologic symptoms within 1 year.
- Patients with total occlusion of the target vessel.
- Patients with evidence of intraluminal thrombus thought to increase the risk of plaque fragmentation and distal embolization.
- Any condition that precluded proper angiographic assessment or made percutaneous arterial access unsafe (e.g., morbid obesity, sustained systolic blood pressure > 180 mmHg).
- Known cardiac sources of emboli.
- Patients with highly calcified lesions resistant to PTA.
- Atherosclerotic disease involving adjoining vessels precluding safe placement of the guiding catheter or sheath.
- The safety and effectiveness of concurrent treatment of lesions in patients with bilateral carotid artery disease have not been established.
Caution should be used if pre-dilating the lesion without embolic protection as this may increase the risk of an adverse outcome.
The BareWire™ Filter Delivery Wire should be kept moistened throughout the procedure.
The Filtration Element can only be positioned proximal to the radiopaque distal section of the Filter Delivery Wire.
To reduce the potential for the liberation of emboli during lesion crossing, the device should be carefully manipulated and not advanced against resistance.
If the Filtration Element moves into the stented vessel segment prior to retrieval, DO NOT RETRIEVE. Use the Retrieval Catheter to gently maneuver the Filtration Element distally until it is situated in an unstented portion of vessel. Retrieval should then proceed.
Maintain proper guiding catheter / sheath support throughout the procedure. Ensure that there is enough distance between the proximal tip of the Filtration Element and the most distal tip of any interventional device to be introduced over the Filter Delivery Wire to avoid engagement. The tip of a balloon catheter or a stent delivery system or an atherectomy device should not contact the Filtration Element. Failure to maintain adequate distance could result in inadvertent Filtration Element movement and Stent Delivery System tip / Filtration Element entanglement and / or Filtration Element /Stent entanglement if guide catheter or sheath prolapse occurs.
In patients requiring the use of antacids and / or H2-antagonists before or immediately after stent placement, oral absorption of antiplatelet agents (e.g., aspirin) may be adversely affected.
The minimum guide catheter or sheath internal diameter is printed on the package label. Do not use a smaller guide catheter or sheath than indicated.
For proper positioning of the filter basket, the vessel distal to the lesion should have an absence of excessive tortuosity and be of adequate length (approximately 4 cm distal to the lesion and proximal to the petrous portion of the vessel).
Do not pull back on the BareWire™ Filter Delivery Wire during advancement.
The Emboshield NAV6 ™ Embolic Protection System is not to be deployed with an access device that uses an integrated leaflet type valve.
Precautions
The device must only be flushed using the 3-ml syringe and flushing tip provided. Use with fixed (passive) hemostatic valves is not recommended.
For best device performance, the guide wire exit notch should remain within the guiding catheter or sheath.
The delivery system is not designed for use with power injection. Use of power injection may adversely affect device performance.
Care must be used when removing the filter basket through a newly deployed stent to maintain filter basket integrity and to avoid disrupting the stent geometry.
To optimize system performance, it is imperative that careful attention is paid to the preparation of the system using the preparation techniques specified in the Instructions For Use. The size of the Filtration Element should be selected to match the diameter of the vessel at the intended site of deployment.
The clinician should take into consideration the slight increase in vessel diameter that may occur after treatment of the lesion.
Precautions to prevent or reduce clotting should be taken when any interventional device is used. Flush or rinse all devices entering the vascular system with heparinized normal saline or alternative anticoagulant, prior to use.
The Emboshield NAV6 ™ Embolic Protection System must be used with a guiding catheter or introducer sheath to maintain adequate support for the BareWire™ Filter Delivery Wire throughout the procedure.
Do not torque the RX Delivery and RX Retrieval Catheters.
The maintenance of blood flow through the device should be observed throughout the procedure by the use of contrast injection.
If excessive debris is collected in the Filtration Element such that distal profusion of dye is significantly reduced or no dye is perfusing past the filter, the Emboshield NAV6 ™ device may have reached its maximum capacity to contain emboli. Remove and replace the
Emboshield NAV6 ™ device. Otherwise, it may be difficult to completely recover all embolic debris and the potential for thrombus formation may increase.
Do not attempt to move the Filtration Element after deployment and prior to the start of retrieval.
Venous access should be available during carotid stenting in order to manage bradycardia and / or hypotension by either pharmaceutical intervention or place of a temporary pacemaker, if needed.
Removal of the BareWire™ Filter Delivery Wire with the Emboshield NAV6 ™ Filtration Element through any interventional devices other than the Emboshield NAV6 ™ RX Retrieval Catheter has not been tested.
The minimum expanded stent internal diameter required for retrieval of a large embolic load is 2.5 mm.
During the insertion of Rapid Exchange catheters through guide catheters or sheaths, careful handling is required to ensure that air is not drawn into the access device. It is therefore recommended that flushing of contrast media (or other fluids) is performed before or after insertion of the catheter, but not while the catheter is within the access device.
Deployment of the Filtration Element, the subsequent deployment of complementary interventional devices over the BareWire™ Filter Delivery Wire, and the retrieval of the Filtration Element should only be performed under fluoroscopic observation.
Potential Adverse Events
As reported in the literature, the following adverse events are potentially associated with carotid stents and embolic protection systems:
- Allergic reaction or hypersensitivity to latex, contrast agent, anesthesia, stent material (Nitinol, Nickel, Titanium) and drug reactions to anticoagulation, or antiplatelet drugs
- Vascular access complications which may require transfusion or vessel repair, including:
- Bleeding (ecchymosis, oozing, hematoma, hemorrhage, retroperitoneal hemorrhage)
- Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation/rupture, and laceration
- Embolism (air, tissue, plaque, thrombotic material or device)
- Thrombophlebitis
- Target artery complications which may require additional intervention, including:
- Total occlusion or abrupt closure
- Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation/rupture
- Embolism (air, tissue, plaque, thrombotic material or device)
- Stenosis or restenosis
- Artery, stent, or filter thrombosis / occlusion thrombosis
- Vessel spasm
- Cardiac arrhythmias (including conduction disorders, atrial and ventricular arrhythmias)
- Cardiac ischemic conditions (including myocardial ischemia, myocardial infarction, and unstable or stable angina pectoris)
- Stroke/Cerebrovascular accident (CVA) and Transient Ischemic Attack (TIA)
- System organ failures:
- Cardio Pulmonary failure
- Renal failure / insufficiency
- Blood cell disorders including heparin induce thrombocytopenia and other coagulopathy
- Hypotension/hypertension
- Peripheral nerve injury
- Other ischemic conditions/infarct
- Infection - local and systemic (including postproceural)
- Chest pain
- Edema/Cerebral edema and fluid overload
- Fever
- Pain, including headache
- Hyperperfusion syndrome
- Other neurologic and systemic complications
- Cerebral hemorrhage
- Death
Any adverse event occurring involving the Emboshield NAV6 ™ Embolic Protection System should be reported immediately to Abbott Vascular, Customer Service: 1-800 227-9902.
MAT-2208561 v3.0
RX Acculink™ Carotid Stent System

Indications
The RX Acculink™ Carotid Stent System, used in conjunction with the Abbott Vascular embolic protection system specified below, is indicated for the treatment of patients at high and standard risk for adverse events from carotid endarterectomy who require carotid revascularization and meet the criteria outlined below:
| High Risk | Standard Risk | |
|---|---|---|
| Embolic Protection System | Abbott Vascular's Accunet™ or Emboshield™ Family | |
| With neurological symptoms | ≥ 50% stenosis of the common or internal carotid artery by ultrasound or angiogram | ≥ 70% stenosis of the common or internal carotid artery by ultrasound or ≥ 50% stenosis of the common or internal carotid artery by angiogram |
| Without neurological symptoms | ≥ 80% stenosis of the common or internal carotid artery by ultrasound or angiogram | ≥ 70% stenosis of the common or internal carotid artery by ultrasound or ≥ 60% stenosis of the common or internal carotid artery by angiogram |
| Reference vessel diameter | Must be within 4.0 mm – 9.00 mm at the target lesion | |
Contraindications
The RX Acculink™ Carotid Stent System is contraindicated for use in:
- Patients in whom anti-coagulant and / or anti-platelet therapy is contraindicated.
- Patients with severe vascular tortuosity or anatomy that would preclude the safe introduction of a guide catheter, sheath, embolic protection system, or stent system.
- Patients with known hypersensitivity to nickel-titanium.
- Patients with uncorrected bleeding disorders.
- Lesions in the ostium of the common carotid artery.
Warnings
Only physicians who have received appropriate training and are familiar with the principles, clinical applications, complications, side effects and hazards commonly associated with carotid stent placement should use this device.
General
Refer to the Instructions for Use supplied with any interventional devices to be used in conjunction with the RX Acculink™ Carotid Stent System for their intended uses, contraindications, and potential complications.
The safety and efficacy of the RX Acculink™ Carotid Stent System have not been demonstrated with embolic protection systems other than Abbott Vascular’s Accunet™ or Emboshield™ family of Embolic Protection Systems (EPS). Refer to the Instructions for Use document for the Embolic Protection System that will be used for specific device instructions.
Clinical study results suggest lower event rates when the RX Acculink™ Carotid Stent System is used in conjunction with an embolic protection device.
The long-term performance (> 3 years) of the Acculink™ Carotid Stent has not been established.
As with any type of vascular implant, infection secondary to contamination of the stent may lead to thrombosis, pseudoaneurysm, or rupture.
Stenting across a major bifurcation may hinder or prevent future diagnostic or therapeutic procedures.
In patients requiring the use of antacids and / or H2-antagonists before or immediately after stent placement, oral absorption of antiplatelet agents (e.g. aspirin) may be adversely affected. The appropriate antiplatelet and anticoagulation therapy should be administered pre- and post-procedure as suggested in these instructions. Special consideration should be given to those patients with recently active gastritis or peptic ulcer disease.
When multiple stents are required, stent materials should be of similar composition.
Patient Selection
The safety and effectiveness of the RX Acculink™ Carotid Stent System have NOT yet been established in patients with the characteristics noted below.
Patient Characteristics
- Patients experiencing acute ischemic neurologic stroke or who experience a stroke within 7 days prior to the procedure.
- Patients with an intracranial mass lesion (i.e., abscess, tumor, or infection) or aneurysm > 5 mm.
- Patients with arteriovenous malformations of the territory of the target carotid artery.
- Patients with coagulopathies.
- Patients with poor renal function who, in the physician’s opinion, may be at high risk for a reaction to contrast medium.
- Patients with perforated vessels evidenced by extravasation of contrast media.
- Patients with aneurysmal dilation immediately proximal or distal to the lesion.
- Pregnant patients or patients under the age of 18.
Lesion Characteristics:
- Patients with evidence of intraluminal thrombus thought to increase the risk of plaque fragmentation and distal embolization.
- Patients whose lesion(s) may require more than two stents.
- Patients with total occlusion of the target vessel.
- Patients with highly calcified lesions resistant to PTA.
Access Characteristics:
- Patients with known peripheral vascular, supra-aortic or internal carotid artery tortuosity that would preclude the use of catheter-based techniques.
- Patients in whom femoral access is not possible.
- Risk of distal embolization may be higher if the RX Acculink™ Carotid System cannot be used in conjunction with an embolic protection system during the carotid stenting procedure.
The safety and effectiveness of concurrent treatment of lesions in patients with bilateral carotid artery disease have not been established.
Device Use
This device is intended for single-use only. Do not reuse. Do not resterilize, as this can compromise device performance and increase the risk of cross contamination due to inappropriate reprocessing.
Do not use the product after the "Use by" date specified on the package.
Do not use the product if the temperature indicator on inner pouch is black.
Maintain the patient’s Activated Clotting Time (ACT) at > 250 seconds throughout RX Acculink™ Carotid Stent System usage to prevent thrombus formation on the device.
Maintain continuous flush while removing and reinserting devices on the guide wire. Perform all exchanges slowly to prevent air embolism or trauma to the artery.
Caution should be used if pre-dilating the lesion without embolic protection as this may increase the risk of an adverse outcome.
Implanting a stent may lead to dissection of the vessel distal and / or proximal to the stent and may cause acute closure of the vessel, requiring additional intervention (carotid endarterectomy, further dilatation, or placement of additional stents).
The stent may cause a thrombus, distal embolization or may migrate from the site of implant down the arterial lumen. Appropriate sizing of the stent to the vessel is required to reduce the possibility of stent migration. In the event of thrombosis of the expanded stent, thrombolysis and PTA should be attempted.
In the event of complications such as infection, pseudoaneurysm or fistulization, surgical removal of the stent may be required.
Overstretching of the artery may result in rupture and life-threatening bleeding.
If a filter-based embolic protection system (EPS) is used, allow for and maintain adequate distance between the RX Acculink™ Carotid Stent System and the EPS to avoid potential filter engagement with the RX Acculink™ Carotid Stent System tip and / or filter entanglement with the deployed stent. If filter engagement and / or entanglement or filter detachment occurs, surgical conversion or additional catheter based intervention may be required.
Ensure optimal positioning of the stent prior to deployment. Once deployment is initiated, the stent cannot be repositioned or recaptured. Stent retrieval methods (use of additional wires, snares and / or forceps) may result in additional trauma to the carotid vasculature and / or the vascular access site. Complications may include death, stroke, bleeding, hematoma or pseudoaneurysm.
Precautions
Stent Handling – Precautions
Carefully inspect the RX Acculink™ Carotid Stent System to verify that the device has not been damaged in shipment. Do not use damaged equipment.
The delivery system has an internal hypotube. Take care to avoid unnecessary handling, which may kink or damage the delivery system. Do not use if device is kinked.
Do not expose the delivery system to organic solvents (e.g. alcohol) as structural integrity and / or function of the device may be impaired.
Do not remove the stent from its delivery system as removal may damage the stent. The stent on the delivery system is intended to perform as a system. If removed, the stent cannot be put back on the delivery system.
The delivery system should not be used in conjunction with other stents.
Special care must be taken not to handle or in any way disrupt the stent on the delivery system. This is most important during catheter removal from packaging, mandrel removal, placement over the guide wire, and advancement through a Rotating Hemostatic Valve (RHV) adapter and guiding catheter hub.
Do not hold the sheath or stent during mandrel removal.
Stent Placement – Precautions
Use with bleedback control hemostatic valves is not recommended.
The RX Acculink™ Carotid Stent System is not compatible with any guide wire larger than 0.014” (0.36 mm).
Leave the safety lock closed until the stent is ready to deploy.
The RX Acculink™ Carotid Stent System must be used with a guiding catheter or introducer sheath to maintain adequate support of the 0.014” (0.36 mm) guide wire throughout the procedure.
For best device performance, the guide wire exit notch should remain within the guiding catheter or sheath.
Ensure the stent system is fully flushed with heparinized saline prior to use. Do not use the delivery system if flush is not observed exiting at the distal end of the sheath.
Do not attempt to pull a partially expanded stent back through the guiding catheter or sheath; dislodgment of the stent from the delivery system may occur.
Venous access should be available during carotid stenting to manage bradycardia and / or hypotension by either pharmaceutical intervention or placement of a temporary pacemaker, if needed.
When catheters are in the body, they should be manipulated only under fluoroscopy. Radiographic equipment that provides high quality images is needed.
The delivery system is not designed for use with power injection. Use of power injection may adversely affect device performance.
If resistance is met during delivery system introduction, the system should be withdrawn and another system used.
Prior to stent deployment, remove all slack from the delivery system.
When more than one stent is required to cover the lesion, or if there are multiple lesions, the distal lesion should be stented first, followed by stenting of the proximal lesion. Stenting in this order obviates the need to cross the proximal stent for placement of the distal stent and reduces the chance of dislodging stents that have already been placed.
If overlap of sequential stents is necessary, the amount of overlap should be kept to a minimum (approximately 5 mm). In no instance should more than 2 stents overlap.
Post-Implant – Precautions
Care must be exercised when crossing a newly deployed stent with other interventional devices to avoid disrupting the stent geometry and placement of the stent.
In the event of thrombosis of the expanded stent, thrombolysis and PTA should be attempted.
MRI Compatibility
Non-clinical testing has demonstrated that the Acculink™ Carotid Stent, in single and overlapped configurations up to 75 mm in length, is MR Conditional. Patients with this implant can be scanned safely under the following conditions:
- Static magnetic field of 1.5 or 3.0 Tesla,
- Spatial gradient of 2500 Gauss/cm (25 T/m),
- Maximum MR system reported whole-body-averaged specific absorption rate (SAR) of 2.0 W/kg (normal operating mode).
Under the scan conditions defined above, the Acculink™ Carotid Stent is expected to product a maximum temperature rise of less than 3.4°C after 16 minutes of continuous scanning.
In non-clinical testing, the image artifact caused by the device extents approximately 5 mm from the Acculink™ Carotid Stent when imaged with a spin echo pulse sequence and a 3.0 Tesla MRI system.
Potential Adverse Events
Based on the literature, and on clinical and commercial experience with carotid stents and embolic protection systems, the following alphabetical list includes possible adverse events associated with use of these devices:
- Allergic reactions to anti-platelet agents / contrast medium
- Aneurysm
- Angina / coronary ischemia
- Arrhythmia
- Arterial occlusion / thrombosis at puncture site or remote site
- Arteriovenous fistula
- Bacteremia or septicemia
- Bleeding from anticoagulant or antiplatelet medications
- Cerebral edema
- Cerebral hemorrhage
- Cerebral ischemia / transient ischemic attack (TIA)
- Congestive heart failure (CHF)
- Death
- Detachment and / or implantation of a component of the system
- Emboli, distal (air, tissue or thrombotic emboli)
- Emergent or urgent endarterectomy surgery (CEA)
- Fever
- Filter thrombosis / occlusion
- Groin hematoma, with or without surgical repair
- Hemorrhage, with or without transfusion
- Hyperperfusion syndrome
- Hypotension / hypertension
- Infection and pain at insertion site
- Ischemia / infarction of tissue / organ
- Myocardial infarction (MI)
- Pain (head, neck)
- Pseudoaneurysm, femoral
- Renal failure / insufficiency
- Restenosis of stented segment
- Seizure
- Severe unilateral headache
- Stent / filter entanglement / damage
- Stent embolization
- Stent malposition
- Stent migration
- Stent thrombosis / occlusion
- Stroke / cerebrovascular accident (CVA)
- Total occlusion of carotid artery
- Vessel dissection, perforation, or rupture
- Vessel spasm or recoil
MAT-2006244 v2.0
XACT™ Carotid Stent System

INDICATIONS
The XACT™ Carotid Stent System (XACT™), used in conjunction with the Emboshield™ family of Embolic Protection System is indicated for the improvement of the lumen diameter of carotid arteries in patients considered at high risk for adverse events from carotid endarterectomy who require percutaneous carotid angioplasty and stenting for occlusive artery disease and meet the criteria outlined below:
Patients with carotid artery stenosis (≥ 50% for symptomatic patients by ultrasound or angiography or ≥ 80% for asymptomatic patients by ultrasound or angiography), located between the origin of the common carotid artery and the intra-cranial segment of the internal carotid artery AND
Patients must have a reference vessel diameter ranging between 4.8 mm and 9.1 mm at the target lesion.
CONTRAINDICATIONS
Contraindications associated with angioplasty must be considered when using the XACT™ Carotid Stent System. These include, but are not limited to:
- Patients in whom anticoagulant and / or antiplatelet therapy is contraindicated.
- Patients with severe vascular tortuosity or anatomy that would preclude the safe introduction of the Guiding Catheter / Introducer Sheath, BareWire™ guide wire, Emboshield™ Delivery Catheter, Filtration Element, and / or Retrieval Catheter.
- Patients with a known hypersensitivity to nickel-titanium.
- Patients with uncorrected bleeding disorders.
- Lesions in the ostium of the common carotid artery.
WARNINGS
Only physicians who have received appropriate training and are familiar with the principles, clinical applications, complications, side effects and hazards commonly associated with carotid interventional procedures should use this device.
General
Refer to instructions supplied with all interventional devices to be used with the XACT™ Carotid Stent System for their intended uses, contraindications, and potential complications.
The safety and efficacy of the XACT™ Carotid Stent System has not been demonstrated with embolic protection systems other than the Emboshield™ Embolic Protection System.
The long-term performance (> 1 year) of the XACT™ Carotid Stent System has not been established.
As with any type of vascular implant, infection secondary to contamination of the stent may lead to thrombosis, pseudoaneurysm, or rupture.
Stenting across a major bifurcation may hinder or prevent future diagnostic or therapeutic procedures.
In patients requiring the use of antacids and / or H2-antagonists before or immediately after stent placement, oral absorption of antiplatelet agents (e.g. aspirin) may be adversely affected.
The appropriate antiplatelet and anticoagulation therapy should be administered pre- and post-procedure as suggested in these instructions. Special consideration should be given to those patients with recently active gastritis or peptic ulcer disease.
When multiple stents are required, stent materials should be of similar composition.
The safety and effectiveness of the XACT™ Carotid Stent System has NOT yet been established in patients with the characteristics noted below.
- Low to moderate risk for adverse events from carotid endarterectomy
- Previously placed stent in target artery.
- Total occlusion of target lesion.
- Angiographically visible thrombus.
- Carotid string sign (a tiny, long segment of contrast in the true lumen of the artery).
- Vessel anatomy precluding the use of the stent system or appropriate positioning of the embolic protection system.
- Presence of carotid artery dissection prior to initiation of the procedure.
- Evidence of a stroke within the previous 30 days.
- History of ipsilateral stroke with fluctuating neurologic symptoms within 1 year.
- History of intracranial hemorrhage within the past 3 months.
- Any condition that precluded proper angiographic assessment or made percutaneous arterial access unsafe, (e.g. morbid obesity, sustained systolic blood pressure > 180 mmHg).
- Contraindication to aspirin, or to clopidogrel AND ticlopidine, or stent material.
- History or current indication of bleeding diathesis or coagulopathy including thrombocytopenia or an inability to receive heparin in amounts sufficient to maintain an activated clot time at > 250 seconds.
- Hemoglobin (Hgb) < 8 gm / dl (unless on dialysis), platelet count < 50,000, INR > 1.5 (irreversible), or heparin-associated thrombocytopenia.
- Known cardiac sources of emboli.
- Atherosclerotic disease involving adjoining vessels precluding safe placement of the guiding catheter or sheath.
- Other abnormal angiographic findings that indicated the patient was at risk of a stroke due to a problem other than that of the target lesion, such as: ipsilateral arterial stenosis greater in severity than the target lesion, cerebral aneurysm, or arteriovenous malformation of the cerebral vasculature.
- Severe dementia.
- Life threatening allergy to contrast media that could not be treated.
- Pregnant patients or patients under the age of 18.
- Patients in whom femoral access is not possible.
- Patients with aneurysmal dilation immediately proximal or distal to the lesion.
The safety and effectiveness of concurrent treatment of lesions in patients with bilateral carotid artery disease have not been established.
PRECAUTIONS
Carefully inspect device components prior to use to verify that they have not been damaged and that the size, shape and condition are suitable for the procedure for which they are to be used. A device or access device which is kinked or damaged in any way should not be used. If pouch is damaged do not use.
Confirm the compatibility of the XACT™ Stent Delivery System with the interventional devices before actual use.
Precautions to prevent or reduce clotting should be taken when any interventional device is used. Flush or rinse all devices entering the vascular system with sterile isotonic heparinized saline prior to use.
Do not remove the stent from its delivery system as removal may damage the stent. The stent and delivery system are intended to be used in tandem. If removed, the stent cannot be put back on the delivery system.
The delivery system should not be used in conjunction with other stents.
To reduce the potential for the liberation of emboli during lesion crossing, the device should be carefully manipulated and not advanced against resistance.
During stent placement, 1.5 cm of vessel should be left between the distal margin of the stent and the Filtration Element. The stent delivery system should not contact the Filtration Element.
Venous access should be available during carotid stenting in order to manage bradycardia and / or hypotension by either pharmaceutical intervention or placement of a temporary pacemaker, if needed.
The device must only be flushed using the 3-ml syringe and flushing tip provided.
The outside diameter of the Outer Sheath is 5.7 Fr. An appropriate sized sheath / guiding catheter should be selected based on this diameter.
Do not use a prepared XACT™ Carotid Stent System if the stent is not fully constrained within the Delivery System.
Do not use if the stent is partially deployed.
If, after preparation, a gap between the catheter tip and the outer sheath exists, rotate the Deployment Actuator in an anti-clockwise direction until the gap is closed.
Advancement and deployment of the XACT™ Carotid Stent System should only be performed under fluoroscopic observation.
Do not advance any component, or section thereof, of the XACT™ Carotid Stent System against significant resistance. The cause of any resistance should be determined via fluoroscopy and remedial action taken.
Do not attempt to reposition the Delivery System once the stent has made contact with the vessel wall.
Do not torque the XACT™ Carotid Stent System.
If more than one stent is required to cover the lesion, or if there are multiple lesions, the distal lesion should be stented first, followed by stenting of the proximal lesion.
If overlap of sequential stents is necessary, the amount of overlap should be kept to a minimum.
MRI Information
Non-clinical testing has demonstrated that the XACT™ Carotid Stent is MR Conditional. It can be scanned safely under the conditions listed in the Instructions for Use.
Potential Adverse Effects
As reported in the literature, the following adverse events are potentially associated with carotid stents and embolic protection systems:
- Abrupt closure
- Allergic reactions
- Aneurysm
- Angina/Coronary ischemia
- Arteriovenous Fistula
- Bacteremia or septicemia
- Bleeding from anticoagulant or antiplatelet medications
- Bradycardia/arrhythmia
- Cerebral edema
- Cerebral hemorrhage
- Congestive Heart Failure
- Death
- Drug reactions
- Embolism (including air and device)
- Emergent or urgent Endarterectomy
- Fever
- Filter thrombosis / occlusion
- Fluid overload
- Groin hematoma, with or without surgical repair
- Hemorrhage or hematoma
- Hemorrhagic stroke
- Headache
- Hypotension
- Hyperperfusion syndrome
- Hypertension
- Infection / sepsis
- Ischemia / infarction of tissue / organ
- Myocardial Infarction
- Other conduction disturbances
- Pain and tenderness
- Pain, infection, or discomfort at the access site
- Pseudoaneurysm
- Renal failure / insufficiency
- Restenosis of the stented artery
- Seizure
- Stent deformation, collapse, fracture, movement of stent, possibly requiring emergency surgery
- Stent / filter entanglement / damage
- Stroke or other neurological complications
- Thromboembolic episodes
- Thrombophlebitis
- Total occlusion of the artery
- Transient ischemic attacks (TIAs)
- Vascular access complications (e.g. loss of pulse, femoral artery pseudoaneurysm and infection)
- Ventricular fibrillation
- Vessel dissection, rupture, or perforation
- Vessel thrombosis (partial blockage)
- Unstable angina pectoris
MAT-2006273 v3.0
