About HeartMate 3 LVAD
The HeartMate 3™ Left Ventricular Assist Device (LVAD) is a mechanical circulatory support device designed for patients with advanced heart failure. With significantly improved outcomes and survival comparable to heart transplant at 2 years, our LVAD device technology is making a better future possible for many patients with advanced heart failure.*1-3 The HeartMate 3 LVAD has advanced the field of LVAD therapy and is setting the standard with innovation and outstanding clinical outcomes.1
The Full MagLev Flow Technology within the HeartMate 3 LVAD maintains gentle blood handling to minimize complications and hemocompatibility-related adverse events.
HeartMate 3 LVAD with Full MagLev Flow Technology Pump offers:
- Fully levitated, self-centering rotor that does not require hydrodynamic or mechanical bearings
- Large, consistent blood flow pathways, which reduces shear stress4
- Intrinsic pulsatility to reduce stasis and minimize thrombus4,5
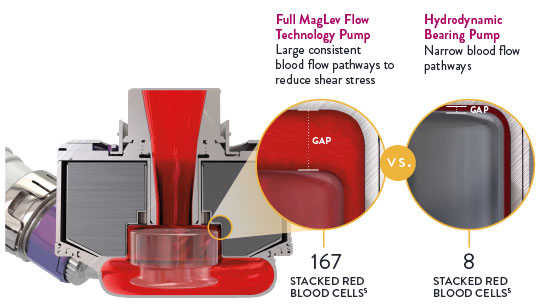
Indications:
The HeartMate 3 Left Ventricular Assist System is indicated for providing short- and long-term mechanical circulatory support (e.g., as bridge to transplant or myocardial recovery, or destination therapy) in adult and pediatric patients with advanced refractory left ventricular heart failure and with an appropriate body surface area.
Contraindications:
The HeartMate 3 Left Ventricular Assist System is contraindicated for patients who cannot tolerate, or who are allergic to, anticoagulation therapy.
HeartMate 3 LVAD Clinical Evidence
There are comprehensive clinical evidence materials available to learn about the survival rate and patient improvement associated with the HeartMate 3 LVAD. For a deep dive into the clinical data, visit the Clinical Evidence and Momentum 3 Trial information.
MOMENTUM 3 5-Year Outcomes
MOMENTUM 3 is the largest LVAD trial ever conducted demonstrating excellent survival and safety outcomes with HeartMate 3™ LVAD.**1
HEARTMATE 3™ LVAD, a proven long-term, life-extending therapy for patients with advanced heart failure
Median survival
exceeding
5 years6

58.4%
survival at
5 years6
Improved Safety Profile7
The ELEVATE Registry7 evaluated the real-world experience of the HeartMate 3 LVAD in a post-approval setting. 5-year extended follow-up showed:
Stroke
10.8% in first 2 years
3.6% in years 2-5
Thrombosis
1.1% at 5 years
All major event types were reduced in the 2-5 years follow-up period compared to 0-2 years, especially hemocompatiblity-related adverse events.
Reimbursement & Coding
For the full resource list, visit Reimbursement & Coding for left ventricular assist devices.
*Based on published data from separate datasets, which may involve different patient populations and other variables. Not a head-to-head comparison. Data presented for informational purposes only.
**HeartMate 3 LVAD demonstrated superiority in event-free survival (primary endpoint) in the MOMENTUM 3 trial compared to HeartMate II™ LVAD.
§For a continuous-flow LVAD in a randomized controlled trial.
References
- Mehra M, Uriel N, Naka Y, et al. A Fully Magnetically Levitated Left Ventricular Assist Device-Final Report. N Engl J Med. 2019;380:1618-1627.
- Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplant Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018;37:1155-1168.
- Teuteberg JJ, Cleveland JC Jr, Cowger J, et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann Thorac Surg. March 2020;109(3):649-660.
- Bourque K, Cotter C, Dague C, et al. Design rationale and preclinical evaluation of the HeartMate 3 Left Ventricular Assist System for hemocompatibility. Am Soc Artificial Int Organs. 2016;62:375-383.
- Bourque K, Dague C, Farrar D, et al. In vivo assessment of a rotary left ventricular assist device-induced artificial pulse in the proximal and distal aorta. Artificial Organs. 2006;30:638-642.
- Mehra MR, Goldstein DJ, Cleveland JC, et al. Five-year outcomes in patients with fully magnetically levitated vs axial-flow left ventricular assist devices in the MOMENTUM 3 randomized trial. JAMA. 2022;328(12):1233-1242. doi:10.1001/jama.2022.16197.
- Schmitto JD, Shaw S, Garbade J, et al. Long-Term Results in Real World Patients Treated with HeartMate 3 LVAD for Advanced Heart Failure: Data from the ELEVATE Registry. European Association for Cardio-Thoracic Surgery (EACTS) Annual Meeting; October 8, 2022; Milan, Italy.
MAT-2012548 v4.0
