About HeartMate 3 LVAD
The HeartMate 3™ Left Ventricular Assist Device (LVAD) is a mechanical circulatory support device designed for patients with advanced heart failure. With significantly improved outcomes and survival comparable to heart transplant at 2 years1-3 and 5 years,4-7 our LVAD technology is making a better future possible for many patients with advanced heart failure.*1-3 The HeartMate 3 LVAD has advanced the field of LVAD therapy and is setting the standard with innovation and outstanding clinical outcomes.1
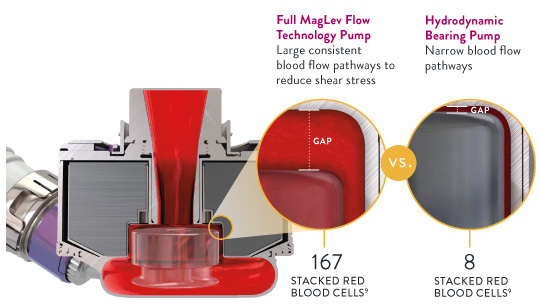
The Full MagLev Flow Technology within the HeartMate 3 LVAD maintains gentle blood handling to minimize complications and hemocompatibility-related adverse events.
HeartMate 3 LVAD with Full MagLev Flow Technology offers
- Fully levitated, self-centering rotor, which does not require hydrodynamic or mechanical bearings
- Large, consistent blood flow pathways, which reduces shear stress8
- Intrinsic pulsatility, which reduces stasis and minimize thrombus8,9
HeartMate 3 LVAD Clinical Evidence
There are comprehensive clinical evidence materials available to learn about the survival rate and patient improvement associated with the HeartMate 3 LVAD. For a deep dive into the clinical data, visit the Clinical Evidence information.
MOMENTUM 3 5-Year Outcomes
MOMENTUM 3 is the largest LVAD trial ever conducted demonstrating excellent survival and safety outcomes with HeartMate 3™ LVAD.**1
HEARTMATE 3™ LVAD, a proven long-term, life-extending therapy for patients with advanced heart failure
Median survival
exceeding
5 years4

58.4%
survival at
5 years4
Improved Safety Profile5
The ELEVATE Registry5 evaluated the real-world experience of the HeartMate 3 LVAD in a post-approval setting. 5-year extended follow-up showed:
Stroke
10.8% in first 2 years
3.6% in years 2-5
Thrombosis
1.1% at 5 years
All major event types were reduced in the 2-5 years follow-up period compared to 0-2 years, especially hemocompatiblity-related adverse events.
This device is commercially available for use in select international markets.
*Based on published data from separate datasets, which may involve different patient populations and other variables. Not a head-to-head comparison. Data presented for informational purposes only.
**HeartMate 3 LVAD demonstrated superiority in event-free survival (primary endpoint) in the MOMENTUM 3 trial compared to HeartMate II™ LVAD.
§For a continuous-flow LVAD in a randomized controlled trial.
References
- Mehra M, Uriel N, Naka Y, et al. A Fully Magnetically Levitated Left Ventricular Assist Device-Final Report. N Engl J Med. 2019;380:1618-1627.
- Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplant Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018;37:1155-1168.
- Teuteberg JJ, Cleveland JC Jr, Cowger J, et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann Thorac Surg. March 2020;109(3):649-660.
- Mehra MR, Goldstein DJ, Cleveland JC, et al. Five-year outcomes in patients with fully magnetically levitated vs axial-flow left ventricular assist devices in the MOMENTUM 3 randomized trial. JAMA. 2022;328(12):1233-1242. doi:10.1001/jama.2022.16197.
- Schmitto JD, Shaw S, Garbade J, et al. Long-Term Results in Real World Patients Treated with HeartMate 3 LVAD for Advanced Heart Failure: Data from the ELEVATE Registry. European Association for Cardio-Thoracic Surgery (EACTS) Annual Meeting; October 8, 2022; Milan, Italy.
- Jorde UP, Saeed O, Koehl D, et al. The Society of Thoracic Surgeons Intermacs 2023 Annual Report: Focus on Magnetically Levitated Devices. Ann Thorac Surg 2024;117(1):33-44. doi:10.1016/j.athoracsur.2023.11.004.
- Nayak A, Hall SA, Uriel N, et al. Predictors of 5-Year Mortality in Patients Managed With a Magnetically Levitated Left Ventricular Assist Device. J Am Coll Cardiol 2023;82(9):771-781. doi:10.1016/j.jacc.2023.05.066.
- Bourque K, Cotter C, Dague C, et al. Design rationale and preclinical evaluation of the HeartMate 3 Left Ventricular Assist System for hemocompatibility. Am Soc Artificial Int Organs. 2016;62:375-383.
- Bourque K, Dague C, Farrar D, et al. In vivo assessment of a rotary left ventricular assist device-induced artificial pulse in the proximal and distal aorta. Artificial Organs. 2006;30:638-642.
MAT-2106243 v2.0
