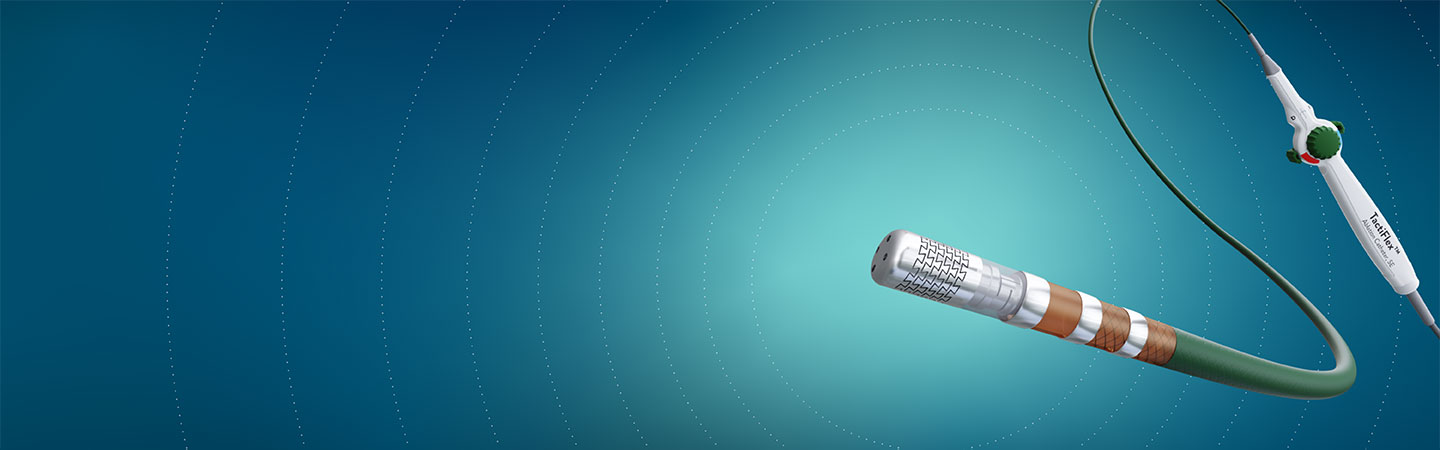
TactiFlex™ Ablation Catheter, Sensor Enabled™
Ablate Efficiently with Predictability and Confidence
- Designed for optimal safety and stability through a flexible laser-cut tip design1
- Confident lesion creation with proven contact-force technology2
- Procedural efficiency3 from intuitive handling and usability with full EnSite™ X EP System integration
Indications:
The TactiFlex™ Ablation Catheter, Sensor Enabled™ is indicated for use in cardiac electrophysiological mapping and for the treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation and concomitant atrial flutter, when used in conjunction with a compatible RF generator and three-dimensional mapping system.
Contraindications:
Do not use for any of the following conditions: recent ventriculotomy or atriotomy heart surgery; prosthetic valves; active systemic infection; use in coronary vasculature; myxoma or intracardiac thrombus, transseptal approach with an interatrial baffle or patch; retrograde trans-aortic approach in patients with aortic valve replacement; patients unable to receive heparin or an acceptable alternative to achieve adequate anticoagulation.
References
- Ambrosius Nick, Fish Jeffrey, Tranter John, Abbott Laboratories. Flexible, kerfed ablation catheter tip provides superior stability in a bench model [abstract]. In: APHRS 2018: Abstract Book; 2018, October 17-18; Taipei, Taiwan. Abstract nr 1170.
- Same contact force technology as in TactiCath Quartz and TactiCath SE, Abbott report 90702721.
- Compared to TactiCath SE and EnSite Precision™. Abbott reports 90486839 and 90773365.
MAT-2302267 v1.0