Our lead portfolio is built on years of proven reliable lead technology. We design for superb deliverability, stability, and performance.
UltiPace™ Pacing Lead with SurGrip™ Technology
Introducing the UltiPace Pacing Lead, newly engineered from Abbott, the first and only provider of stylet-driven leads that are FDA approved for left bundle branch area pacing (LBBAP) with a robust distal lead tip design.* LBBAP performance features include resistance to distal fatigue failure,1 zero helix retraction1 when used with the Helix Locking Tool, and improved torque transmission.1 An enhanced proximal lead end provides increased abrasion and lead crush resistance with 6F compatibility.2

Durata™ Defibrillation Lead
Designed to offer high-performance handling and more control at implant, the Durata Defibrillation Lead is a cardiac lead that has a proven structural design for reliability and long-term durability.3,4 The Durata lead is designed to deal with challenging ICD lead cases, including cases requiring multi-lead systems, lead replacement, or with patients who have small or tortuous veins.
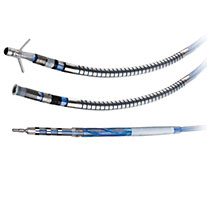
Quartet™ Quadripolar LV Lead
The Quartet Quadripolar LV Lead offers superb deliverability with exceptional stability and performance. With four electrodes and up to 14 pacing configurations, the quadripolar system enables left ventricular (LV) pacing at the preferred site without compromising lead stability.5
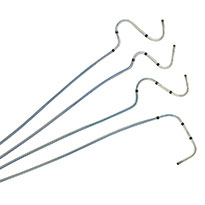
Tendril™ STS Pacing Lead
The Tendril STS Pacing Leads have helped improve patient health and restore quality of life to thousands across the globe. Compatible with a 6F introducer, the Tendril™ STS Pacing Lead has a thin profile, soft silicone tip, and abrasion-resistant Optim™ lead insulation.4

Powering Hearts Beat to Beat
Sign up to hear about our technology, education opportunities, and more.
Read the Latest Blog Article
Stay up to date with recent news, product highlights, and case studies.
References
*Robust distal lead tip without a flexible zone in distal 14.5 mm.
- Abbott. Data on File, Engineering Technical Report. Item: 90994484 Ver D.
- Abbott. Data on File. Item: 91010074 Ver A.
- Jenney C, Tan J, Karicherla A, et al. A new insulation materials for cardiac leads with potential for improved performance. Heart Rhythm. 2005;2(5 supp):S318-S319. DOI: 10.1016/j.hrthm.2005.02.1004.
- St. Jude Medical. Test Report No. 50009843.
- Data compiled from clinical study results, on file at St. Jude Medical in Report 60034670.
Indications, Safety and Warnings
UltiPace Pacing Leads
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
Indications: UltiPace™ leads are indicated for use in combination with a compatible pacemakers, implantable cardioverter defibrillator (ICDs) or cardiac resynchronization therapy (CRT-P/CRT-D) to provide sensing and pacing for the management of chronic symptomatic bradycardia and various atrioventricular conduction abnormalities in patients who experience syncope, presyncope, fatigue, disorientation due to arrhythmia/ bradycardia, or any combination of these symptoms. The UltiPace leads are implanted transvenously in either the right atrium, the right ventricle or the left bundle branch area.
Contraindications: UltiPace™ leads are contraindicated: in the presence of tricuspid atresia (if the lead is to be positioned in the right ventricle or left bundle branch area), for patients with mechanical tricuspid valves (if the lead is to be positioned in the right ventricle or left bundle branch area), in patients who are expected to be hypersensitive to a single dose of one milligram of dexamethasone sodium phosphate.
Adverse Events: Potential adverse effects and their categories associated with the use of UltiPace™ leads are the same as with the use of other active fixation leads and include: Arrhythmia (Accelerated arrhythmia, Induced atrial ectopy or arrhythmias, Induced atrioventricular or bundle branch block, Induced ventricular ectopy or asystole, Myocardial irritability), Cardiac perforation (Cardiac tamponade, Pericardial Effusion, Pericarditis, Septal perforation), Death, Embolism (Air embolus, Dislodgement of intracardiac thrombus, intravascular foreign body), Extra-cardiac stimulation, Heart failure (Right ventricular decompensation, Tricuspid valve dysfunction/Tricuspid valve regurgitation/insufficiency), Hypersensitivity (Hypersensitivity, including local tissue reaction or allergic reaction), Infection (Endocarditis), Lead revision or reprogramming resulting from, but not limited to, loss of pacing and/ or sensing (Electrical malfunction of the lead, Lead dislodgement, Lead dysfunction (sensing/threshold Issue), Mechanical malfunction of the lead), Lung perforation (Hemothorax, Pneumothorax), Pulmonary edema, Prolonged exposure to fluoroscopic radiation, Respiratory compromise, Tricuspid value perforation, Vascular injury (Arterial perforation, Arteriovenous fistula, Coronary sinus or coronary vein perforation/dissection, Hemorrhage/ Hematoma at device site, Venous perforation, Septal hematoma), Vascular thrombosis/ stenosis/ occlusion. The physician should discuss the patient's potential adverse events with them.
Refer to the User’s Manual for detailed indications, contraindications, warnings, precautions and potential adverse events.
Durata
7120, 7121, 7122
Rx Only
Indications/Intended Use: The Durata™ defibrillation leads are indicated for use in combination with implantable cardioverter defibrillators (ICDs) or cardiac resynchronization therapy-defibrillators (CRT-Ds) to provide sensing, pacing and cardioversion/defibrillation therapy for the treatment of bradyarrhythmia's and life-threatening tachyarrhythmias in patients who are at risk from sudden cardiac death. They are implanted transvenously in the right ventricle.
Contraindications: Durata™ Models 7120, 7121, and 7122 leads are contraindicated in the following: Patients with tricuspid valvular disease or a mechanical tricuspid valve. Patients with ventricular tachyarrhythmias resulting from transient or correctable factors such as drug toxicity, electrolyte imbalance, or acute myocardial infarction. Patients for whom a single dose of 1.0 mg of dexamethasone sodium phosphate is contraindicated.
Potential Adverse Events: The following lists potential adverse effects and their categories as applicable when using a high voltage lead. Arrhythmia (Arrhythmia acceleration, Induced atrial ectopy or arrhythmias, Induced atrioventricular or bundle branch block, Induced ventricular ectopy or arrhythmias, Myocardial irritability), Cardiac perforation (Cardiac tamponade, Perforation of the myocardium, Pericardial effusion), Death, Embolism (Air embolism, Intravascular foreign body, Dislodgement of intracardiac thrombus), Extra‑cardiac stimulation (Diaphragmatic stimulation, Stimulation of phrenic nerve), Heart failure (Cardiogenic shock, Right ventricular decompensation, Pacing‑induced cardiomyopathy, Tricuspid valve dysfunction), Hemorrhage, (Acute hemorrhage/bleeding), Hypersensitivity (Hypersensitivity, including local tissue reaction or allergic reaction), Infection (Endocarditis, Pericarditis), Lead revision or reprogramming (Electrical malfunction of the lead, Lead dislodgement, Lead dysfunction (sensing/threshold issue), Mechanical malfunction of the lead, Inappropriate defibrillation therapy), Pleural perforation (Hemothorax, Pneumothorax), Pocket Erosion (Erosion of the skin, Extrusion), Prolonged exposure to fluoroscopic radiation, Seroma (Fluid accumulation), Tissue necrosis, Tricuspid valve perforation, Vascular perforation (Arteriovenous fistula, Arterial perforation, Coronary sinus or coronary vein perforation, Hematoma, Venous perforation), Vascular Thrombosis (Venous thrombosis and/or obstruction, Venous occlusion). The physician should discuss the patient’s potential adverse events with them.
Refer to the User’s Manual for detailed indications, contraindications, warnings, precautions and potential adverse events.
Durata
7120Q, 7121Q, and 7122Q
Rx Only
Indications/Intended Use: The Durata™ Models 7120Q, 7121Q, and 7122Q leads are 7 French, transvenous, steroid eluting, bipolar, DF4 compatible (single connector with four electrical terminals), active fixation leads intended for permanent sensing and pacing of the right ventricle and the delivery of cardioversion/defibrillation therapy when used with a compatible Abbott Medical pulse generator with a DF4-LLHH lead receptacle designation.
Contraindications: Durata™ Models 7120Q, 7121Q, and 7122Q leads are contraindicated in the following: Patients with tricuspid valvular disease or a mechanical tricuspid valve. Patients with ventricular tachyarrhythmias resulting from transient or correctable factors such as drug toxicity, electrolyte imbalance, or acute myocardial infarction. Patients for whom a single dose of 1.0 mg of dexamethasone sodium phosphate is contraindicated. For use with extra firm (red color knob) stylets.
Potential Adverse Events: Possible adverse events associated with the use of transvenous lead systems include, but are not limited to, those summarized in as follows. Refer to the appropriate pulse generator manual for additional complications and precautions specific to the pulse generator. Dislodgement, breaching of the lead insulation, connector fracture, poor connection to the pulse generator, electrode fracture, or conductor is continuity (Intermittent or continuous loss of sensing, possibly resulting immunodetection of arrhythmia; oversensing of artifact, possibly causing inappropriate delivery of therapy from the pulse generator; intermittent or continuous loss of defibrillation, cardioversion, or pacing therapy; possible muscle or nerve stimulation in the pocket area; intermittent or continuous loss of cardioversion/defibrillation therapy, sensing, or pacing therapies.), Cardiac perforation (Intermittent or continuous loss of sensing, cardiac tamponade, hemorrhage, pneumothorax, or loss of contractility), Venous perforation (Acute hemorrhage (may not be readily apparent), hemothorax, pneumothorax, or cardiac tamponade), Myocardial irritability (Premature ventricular contractions, supraventricular and ventricular tachyarrhythmias, postoperative heart failure), Transvenous implantation procedure (Air embolism), Chronic (> 3 months) implantation (Venous thrombosis and/or obstruction, tissue necrosis, skin erosion, tricuspid valve dysfunction, chronic mechanical stimulation of the heart), Contamination (Infection requiring removal of lead system, pulse generator, or both), Post-shock rhythm disturbances (Post-shock bradycardia or supraventricular arrhythmias, conduction disturbances), Threshold elevation or exit block (Loss of efficacy of defibrillation, cardioversion, or pacing therapy), Shunting or insulating of current during defibrillation with internal or external paddles (Increased external defibrillation energy and/or repositioning of paddles required).
Refer to the User’s Manual for detailed indications, contraindications, warnings, precautions and potential adverse events.
Durata™ Defibrillation Leads Model
7170 Model 7171
Rx Only
Indications/Intended Use: The Durata™ Models 7170 and 7171 transvenous leads are indicated for use with compatible pulse generators (refer to the applicable defibrillator manual for system indications). They provide pacing and sensing and deliver cardioversion/defibrillation therapy to the heart.
Contraindications: Durata™ Models 7170 and 7171 leads are contraindicated in the following:
▪ Patients with tricuspid valvular disease or a mechanical tricuspid valve.
▪ Patients with ventricular tachyarrhythmias resulting from transient or correctable factors such as drug toxicity, electrolyte imbalance, or acute myocardial infarction.
▪ Patients for whom a single dose of 1.0 mg of dexamethasone sodium phosphate is contraindicated.
Potential Adverse Events: Possible adverse events associated with the use of transvenous lead systems include, but are not limited to Dislodgement, breaching of the lead insulation, connector fracture, poor connection to the pulse generator, electrode (Intermittent or continuous loss of sensing, possibly resulting in nondetection of arrhythmia; oversensing of artifact, possibly causing inappropriate delivery of therapy from the pulse generator; intermittent or continuous loss of defibrillation, cardioversion, or pacing therapy; possible); fracture, or conductor discontinuity (muscle or nerve stimulation in the pocket area; intermittent or continuous loss of cardioversion/defibrillation therapy, sensing, or pacing therapies.); Cardiac perforation (Intermittent or continuous loss of sensing, cardiac tamponade, hemorrhage, pneumothorax, or loss of contractility); Venous perforation (Acute hemorrhage (may not be readily apparent), hemothorax, pneumothorax, or cardiac tamponade); Myocardial irritability (Premature ventricular contractions, supraventricular and ventricular tachyarrhythmias, postoperative heart failure); Transvenous implantation procedure (Air embolism); Chronic (> 3 months) implantation (Venous thrombosis and/or obstruction, tissue necrosis, skin erosion, tricuspid valve dysfunction, chronic mechanical stimulation of the heart); Contamination (Infection requiring removal of lead system, pulse generator, or both); Post-shock rhythm disturbances (Post-shock bradycardia or supraventricular arrhythmias, conduction disturbances); Threshold elevation or exit block (Loss of efficacy of defibrillation, cardioversion, or pacing therapy) Shunting or insulating of current during defibrillation with internal or external paddles (Increased external defibrillation energy and/or repositioning of paddles required).
Refer to the User’s Manual for detailed indications, contraindications, warnings, precautions and potential adverse events.
Durata™ Defibrillation Leads Models
Models 7170Q, 7171Q, 7172Q
Rx Only
Indications/Intended Use: Durata™ Models 7170Q, 7171Q, and 7172Q leads are 7 French, transvenous, steroid eluting, bipolar, DF4 compatible (single connector with four electrical terminals), passive fixation leads intended for permanent sensing and pacing of the right ventricle and the delivery of cardioversion/defibrillation therapy when used with a compatible Abbott Medical pulse generator with a DF4‑LLHH lead receptacle designation.
Contraindications: Durata™ Models 7170Q, 7171Q, and 7172Q leads are contraindicated in the following:
▪ Patients with tricuspid valvular disease or a mechanical tricuspid valve.
▪ Patients with ventricular tachyarrhythmias resulting from transient or correctable factors such as drug toxicity, electrolyte imbalance, or acute myocardial infarction.
▪ Patients for whom a single dose of 1.0 mg of dexamethasone sodium phosphate is contraindicated.
Potential Adverse Events: Dislodgement, breaching of the lead insulation, connector fracture, poor connection to the pulse generator, electrode fracture, or conductor discontinuity. (Intermittent or continuous loss of sensing, possibly resulting in nondetection of arrhythmia oversensing of artifact, possibly causing inappropriate delivery of therapy from the pulse generator; intermittent or continuous loss of defibrillation, cardioversion, or pacing therapy; possible muscle or nerve stimulation in the pocket area; intermittent or continuous loss of cardioversion/defibrillation therapy, sensing, or pacing therapies.); Cardiac perforation (Intermittent or continuous loss of sensing, cardiac tamponade, hemorrhage, pneumothorax, or loss of contractility); Venous perforation (Acute hemorrhage (may not be readily apparent), hemothorax, pneumothorax, or cardiac tamponade); Myocardial irritability (Premature ventricular contractions, supraventricular and ventricular tachyarrhythmias, postoperative heart failure); Transvenous implantation procedure (Air embolism); Chronic (> 3 months) implantation (Venous thrombosis and/or obstruction, tissue necrosis, skin erosion, tricuspid valve dysfunction, chronic mechanical stimulation of the heart); Contamination (Infection requiring removal of lead system, pulse generator, or both); Post-shock rhythm disturbances (Post-shock bradycardia or supraventricular arrhythmias, conduction disturbances); Threshold elevation or exit block (Loss of efficacy of defibrillation, cardioversion, or pacing therapy); Shunting or insulating of current during defibrillation with internal or external paddles (Increased external defibrillation energy and/or repositioning of paddles required).
Refer to the User’s Manual for detailed indications, contraindications, warnings, precautions and potential adverse events.
Quartet
Family of LV Leads
Rx Only
Brief Summary: Prior to using these devices, please review the User’s Manual for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
Indications and Usage: The Quartet™ leads are 5.1 French, transvenous, steroid eluting, quadripolar, IS4 compatible (single connector with four electrical terminals), passive fixation leads intended for permanent sensing and pacing of the left ventricle when used with a compatible Abbott Medical biventricular system with an IS4‑LLLL lead receptacle designation.
Contraindications: he use of the Quartet LV lead is contraindicated in patients who:
- Are expected to be hypersensitive to a single dose of 1.0 mg of dexamethasone sodium phosphate.
- Are unable to undergo an emergency thoracotomy procedure.
- Have coronary venous vasculature that is inadequate for lead placement, as indicated by venogram.
Adverse Events: Potential adverse events associated with the use of left ventricular leads include: Allergic reaction to contrast media, Body rejection phenomena, Cardiac/coronary sinus dissection , Cardiac/coronary sinus perforation, Cardiac tamponade, Coronary sinus or cardiac vein thrombosis, Death, Endocarditis, Excessive bleeding, Hematoma/seroma, Induced atrial or ventricular arrhythmias, Infection, Lead dislodgment, Local tissue reaction; formation of fibrotic tissue, Loss of pacing and/or sensing due to dislodgment or mechanical malfunction of the pacing lead, Myocardial irritability, Myopotential sensing, Pectoral/diaphragmatic/phrenic nerve stimulation, Pericardial effusion, Pericardial rub, Pneumothorax/hemothorax, Prolonged exposure to fluoroscopic radiation, Pulmonary edema Renal failure from contrast media used to visualize coronary veins, Rise in threshold and exit block, Thrombolytic or air embolism, Valve damage Performance of a coronary sinus venogram is unique to lead placement in the cardiac venous system, and carries risks. Potential complications reported with direct subclavian venipuncture include hemothorax, laceration of the subclavian artery, arteriovenous fistula, neural damage, thoracic duct injury, cannulation of other vessels, massive hemorrhage, and rarely, death.
Tendril STS Pacing Leads
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
Indications: Tendril™ STS leads are indicated for use in combination with a compatible pacemaker, implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy (CRT-P/CRT-D) device to provide sensing and pacing for the management of chronic symptomatic bradycardia and various atrioventricular conduction abnormalities in patients who experience syncope, presyncope, fatigue, or disorientation due to arrhythmia/bradycardia, or any combination of these symptoms. The Tendril STS leads are implanted transvenously in either the right atrium, the right ventricle or the left bundle branch area.
Contraindications: Tendril™ STS Model 2088TC leads are contraindicated: in the presence of tricuspid atresia (if the lead is to be positioned in the right ventricle or left bundle branch area), for patients with mechanical tricuspid valves (if the lead is to be positioned in the right ventricle or left bundle branch area), in patients who are expected to be hypersensitive to a single dose of one milligram of dexamethasone sodium phosphate.
Adverse Effects: Potential adverse effects and their categories associated with the use of Tendril STS leads are the same as with the use of other active fixation leads and include: Arrhythmia (Accelerated arrhythmia, Induced atrial ectopy or arrhythmias, Induced atrioventricular or bundle branch block, Induced ventricular ectopy or asystole, Myocardial irritability), Cardiac perforation (Cardiac tamponade, Pericardial Effusion, Pericarditis, Septal perforation), Death, Embolism (Air embolus, Dislodgement of intracardiac thrombus, intravascular foreign body), Extra-cardiac stimulation, Heart failure (Right ventricular decompensation, Tricuspid valve dysfunction/Tricuspid valve regurgitation/insufficiency), Hypersensitivity (Hypersensitivity, including local tissue reaction or allergic reaction), Infection (Endocarditis), Lead revision or reprogramming resulting from, but not limited to, loss of pacing and/ or sensing (Electrical malfunction of the lead, Lead dislodgement, Lead dysfunction (sensing/threshold Issue), Mechanical malfunction of the lead),Lung perforation (Hemothorax, Pneumothorax), Pulmonary edema, Prolonged exposure to fluoroscopic radiation, Respiratory compromise, Tricuspid value perforation, Vascular injury (Arterial perforation, Arteriovenous fistula, Coronary sinus or coronary vein perforation/dissection, Hemorrhage/ Hematoma at device site, Venous perforation, Septal hematoma), Vascular thrombosis/ stenosis/ occlusion. The physician should discuss the patient's potential adverse events with them.
Refer to the User’s Manual for detailed indications, contraindications, warnings, precautions and potential adverse events
MAT-2007652 v3.0

