The Only Acute Device Approved as a 30-Day VAD* – Cleared for ECMO***
With the addition of the new ECMO indication for the CentriMag™ Blood Pump, the CentriMag™ System addresses the broadest spectrum of clinical challenges in a single platform allowing for escalation and de‑escalation of therapy1-3
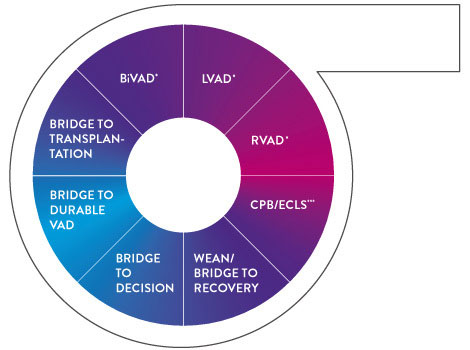
Powered by Full Maglev™ Flow Technology, the Centrimag Pump Enables Excellent Hemodynamics4,5 and Optimal Clinical Outcomes1
See the Centrimag Pump in Action
Our Reliability is backed by our Unmatched Legacy of Experience
Four
Our device is proven safe by four FDA-approved clinical studies*
400
Our device has been cited in over 400 peer-reviewed articles9
Excellent Outcomes
Our Full MagLev Flow Technology drives the same market-leading Heartmate 3™ LVAD with excellent clinical outcomes10
*PMA approval for 30-day use of CentriMag™ System excludes: PediMag™ Blood Pump and any other pediatric components or accessories.
**As part of a VA-ECMO circuit
***ECMO clearance for >6-hour use is indicated for the CentriMag™ Blood Pump to be used with: CentriMag™ Console, CentriMag™ Motor, Mag Monitor, and Flow Probe. ECMO clearance for the CentriMag™ Blood Pump is for adult use only and excludes: CentriMag™ Drainage Cannula and CentriMag™ Return Cannula.
References
- Takeda K, Garan AR, Ando M, et al. Minimally invasive CentriMag ventricular assist device support integrated with extracorporeal membrane oxygenation in cardiogenic shock patients: a comparison with conventional CentriMag biventricular support configuration. Eur J Cardiothorac Surg. 2017;52:1055–61.
- Worku B, Pak S, Patten D, et al. The CentriMag ventricular assist device in acute heart failure refractory to medical management. J Heart Lung Transplant. 2012;31:611–7.
- Takayama H, Soni L, Kalesan B, et al. Bridge-to-decision therapy with a continuous-flow external ventricular assist device in refractory cardiogenic shock of various causes. Circ Heart Fail. 2014;7;799-806.
- den Uil CA, Akin S, Jewbali LS, et al. Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2017;52:14–25.
- John R, Massey HT, Griffith BP, et al. Outcomes of a multicenter trial of the Levitronix CentriMag ventricular assist system for short-term circulatory support. J Thoracic Cardiovasc Surg. 2011;141:932-939.
- John R, Liao K, Lietz K, et al. Experience with the Levitronix CentriMag circulatory support system as a bridge to decision in patients with refractory acute cardiogenic shock and multisystem organ failure. J Thorac Cardiovasc Surg. 2007;134:351-358.
- Aziz TA, Singh G, Popjes E, et al. Initial experience with CentriMag extracorporeal membrane oxygenation for support of critically ill patients with refractory cardiogenic shock. J Heart Lung Transplant. 2010;29:66-71.
- Bhama JK, Kormos RL, Toyoda Y, et al. Clinical experience using the Levitronix CentriMag system for temporary right ventricular mechanical circulatory support. J Heart Lung Transplant. 2009;28:971-976.
- Data on file. Abbott; 2019.
- Mehra M, Uriel N, Naka Y, et al. A Fully Magnetically Levitated Ventricular Assist Device-Final Report. N Engl J Med. 2019;380:1618-1627.
MAT-2012740 v2.0
