REPLACE. RESTORE. RECLAIM.
Tendyne™ transcatheter mitral valve replacement (TMVR) improves your patients' function and quality of life by offering select patients with symptomatic, significant MR (≥ grade 3) a less invasive mitral valve replacement option over conventional mitral valve surgery. Tendyne TMVR provides symptom relief by eliminating MR.1
- Beating heart procedure without the need for cardiopulmonary bypass or rapid pacing
- Complete MR elimination in 99% of patients at 30 days1
- Sustained MR elimination in 93% of patients at 2 years1
- 96% technical success rate1,*
- System is fully repositionable and retrievable at any point intraprocedurally
- Significant improvement in symptom and quality-of-life measures1
- Low mean mitral gradient of 3.2 mm Hg at 2 years1
*Technical success per Mitral Valve Academic Research Consortium (MVARC).
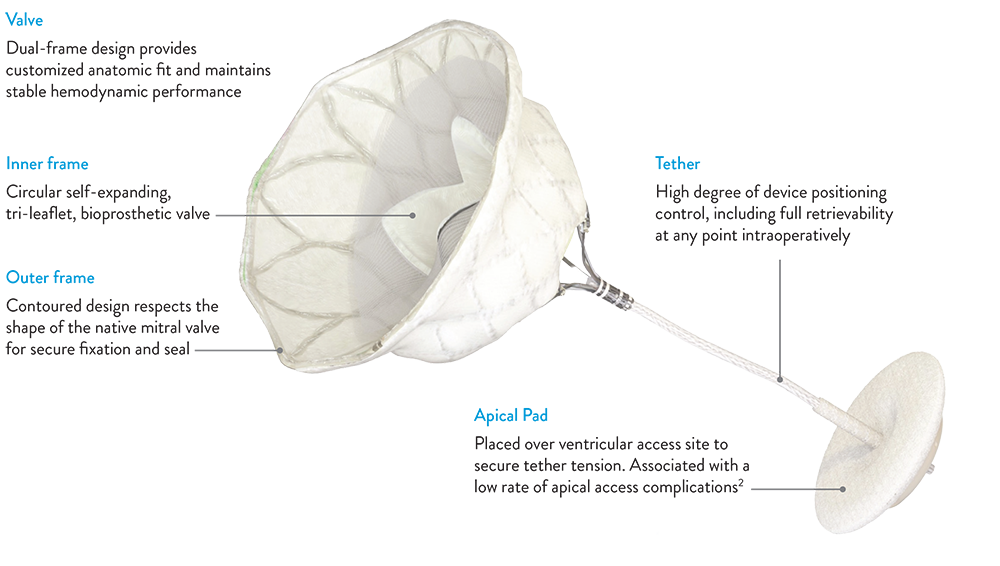
Designed for the mitral anatomy to eliminate MR
Learn more about Tendyne™ TMVR by visiting the Abbott Structural Heart website
Ordering Information - Tendyne™ TMVR
Valve Product Specifications
| Model | Valve Number | Catalog Number | Device AP (mm) | Device IC (mm) | Device Perimeter (mm) | EOA (cm2) |
|---|---|---|---|---|---|---|
| SP | 39M | TENDV-SP-39M | 38.5 | 50.5 | 156 | 3.0 |
| LP | 29S | TENDV-LP-29S | 29.0 | 42.5 | 119 | 2.2 |
| 29L | TENDV-LP-29L | 29.0 | 47.5 | 129 | ||
| 33S | TENDV-LP-33S | 32.5 | 43.5 | 130 | ||
| 35M | TENDV-LP-35M | 34.5 | 48.5 | 144 |
Implantation and Retrieval System Product Specifications
| Description | Catalog Number | Approximate Dimensions (cm) |
|---|---|---|
| Tendyne™ Loading System | TENDV-LS | 15 x 8 x 49 |
| Tendyne™ Delivery System* | TENDV-DS | 6.5 x 7.5 x 89 |
| Tendyne™ Pad Position System | TENDV-PS | 5 x 7.5 x 38 |
| Tendyne™ Gen 2 Retrieval System* | TENDV-RS2 | 15 x 8 x 45 |
| Tendyne 2 Lb. Weight | TENDV-WT | N/A |
| Tendyne Stand | TENDV-ST | N/A |
| Tendyne Stand Components | TENDV-SC | N/A |
*Sheath ID for the delivery system and retrieval system is 36F.
References
- Muller D, et al, 2-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Symptomatic Mitral Regurgitation, J Am Coll Cardiol, 2021;78(19)1847-1859
- Apical Pad: Sorajja P, Moat N, Badhwar V, et al. Initial feasibility study of a new transcatheter mitral prosthesis: the first 100 patients. J Am Coll Cardiol. 2019;73(11):1250-1260.
- Tendyne IFU (EU MDR version)
MAT-2002383 v5.0 | Item approved for OUS use only.
