Acute Clinical Success
TactiFlex™ Ablation Catheter, Sensor Enabled™ acute IDE study results1,2
High-power (time-averaged power setting 40-50W) versus low-power (time-averaged power setting < 40W)
99.4%

Acute Success*
80.5%
81.8% High-Power
77.3% Low-Power
First-Pass PVI Success**
2.4%
1.3% High-Power
5.2% Low-Power
Rate of Repeat Ablation in Blanking Period
4.3%***
4.1% High-Power
5.2% Low-Power
Subjects with Primary Safety Endpoint Event at 90 Days
Shorter ablation and procedure times, less fluoro and irrigation3,4
All power levels up to 50W
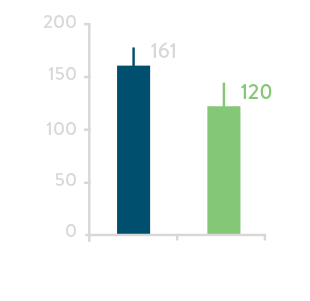
Total Procedure Time****
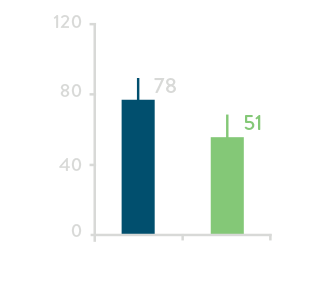
Total PV Ablation Time****
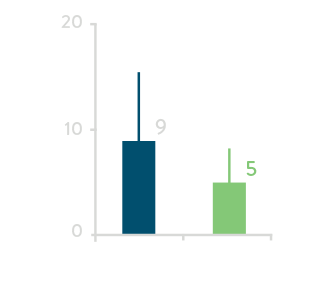
Total Fluoroscopy Time
Irrigation Fluid Volume
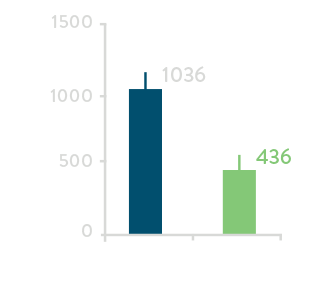
Comparing acute results from high- and low-power groups1
Total Procedure Time****
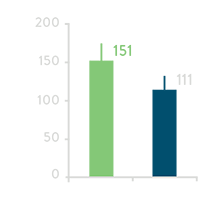
Total RF time for PV Ablation
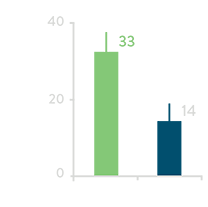
Total Fluoroscopy Time
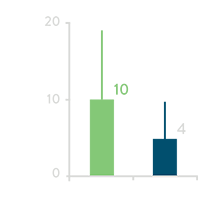
Total Irrigation Fluid Volume
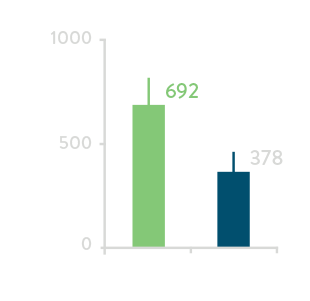
LP = low-power (time-averaged power <40W)
HP = high-power (time-averaged power 40-50W)
This device is commercially available for use in select international markets.
* Isolation of all pulmonary veins, confirmed by entrance block, by the end of the procedure.
** The percentage of treated patients with isolation of all pulmonary veins after a minimum 20-minute waiting period.
*** Endpoint population (main study)
**** Includes 20-minute waiting period.
References
- CL1017540 TactiFlex PAF IDE PMA Report.
- CL1019990 TactiFlex PAF IDE As Treated Repeat Procedure Details.
- CL1021674 TactiFlex and TactiSense Additional Analysis
- CL1004955 TactiSense PMA Report. TactiSense is the PAF IDE study for TactiCath Ablation Catheter, Sensor Enabled.
MAT-2208527 v3.0