Ablate Efficiently with Predictability and Confidence
The first and only contact force catheter with a flexible tip.
Designed for optimal safety and stability, confident lesion creation and procedural efficiency,1 the TactiFlex™ Ablation Catheter, Sensor Enabled™ elevates ablation procedures through:
- A proprietary tip design and full integration with the revolutionary EnSite™ X EP System
- Superior tip stability2,* and excellent signal quality3 during ablation
- Predictability with intuitive contact force arrow and deflection direction indicator available with the EnSite X EP System4
TactiFlex Ablation Catheter, Sensor Enabled Technology Animation
See a simulation of the flexible tip, stability and contact force measurement through fiber-optic technology.
Perform High-Power Ablation** with Safety and Stability, Confidence and Efficiency
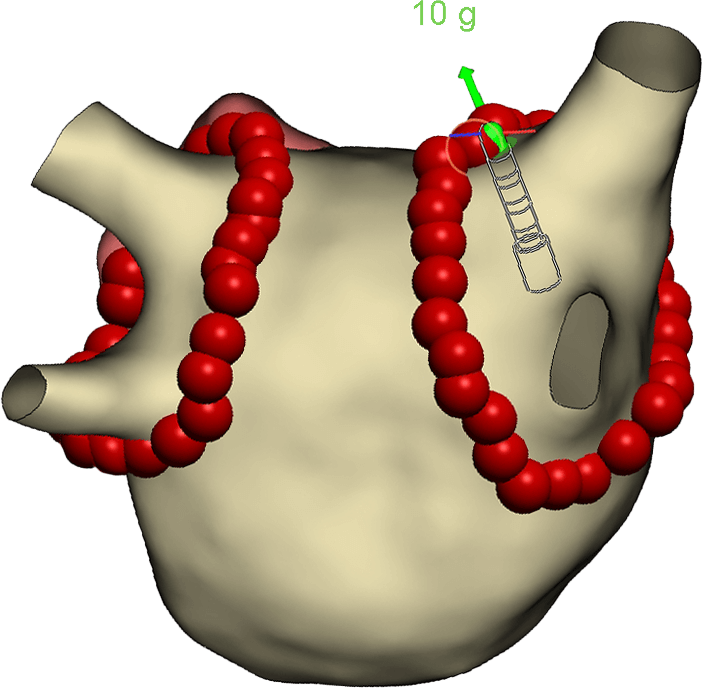
Designed for Optimal Safety and Stability
- Experience superior stability with a unique, flexible, laser-cut tip - up to 2X greater stability than conventional 56-hole catheters2
- Apply directed irrigation flow5 to the tip-tissue interface, effectively cooling tissue for safe transmural ablation
- Lower irrigation rate*** while ablating up to 50W to reduce fluid loading
Confident Lesion Creation
- Ablate with the proven performance of the Abbott contact force platform6
- Optimize contact force measurement through fiber-optic technology
- High resolution (1g) real-time (50Hz) force display with thermal compensation and no interference from other catheters
Procedural Efficiency1 from intuitive handling and usability with full EnSite X EP System integration
- Reduce PVI ablation times by as much as 70%**** with high-power short-duration ablation7
- Usability and predictability with intuitive contact force arrow and deflection direction indicator4
- Monitor lesion creation with excellent electrogram signal quality even during RF3
This device is commercially available for use in select international markets.
*Compared to conventional 56-hole catheters
**Up to 50W
*** Compared to TactiCath SE flow rates and compared to SmartTouch SF flow rate above 30W ablation
**** Compared to standard power ablation (50W vs. 30W)
References
- CL1017540 TactiFlex PAF IDE PMA Report
- Ambrosius Nick, Fish Jeffrey, Tranter John, Abbott Laboratories. Flexible, kerfed ablation catheter tip provides superior stability in a bench model [abstract]. In: APHRS 2018: Abstract Book; 2018, October 17-18; Taipei, Taiwan. Abstract nr 1170.
- Abbott report 90486839.
- Compared to TactiCath SE and EnSite Precision™. Abbott reports 90486839 and 90773365.
- Abbott report 90906650.
- Same contact force technology as in TactiCath Quartz and TactiCath SE, Abbott report 90702721.
- Ptaszek LM et al. Safe and effective delivery of high-power, short-duration radiofrequency ablation lesions with a novel, flexible-tip radiofrequency ablation catheter [abstract]. In: Heart Rhythm 2021: July 28-31, Boston. Heart Rhythm Volume 18, Issue 9, Supplement. Elsevier; 2021. Abstract nr B-PO02-109.
MAT-2208526 v2.0