Clinical Evidence for Diamondback 360™ Peripheral Orbital Atherectomy System
Despite disease complexities, Diamondback 360™'s dual-action addresses clinical challenges to optimize patient outcomes. The debulking of intimal calcium and fracturing of medial calcium offers a complete solution to your vessel preparation needs. Specifically designed to tackle a wide range of disease complexities, Diamondback 360™ navigates clinical challenges safely and efficiently, providing long-term, durable outcomes.
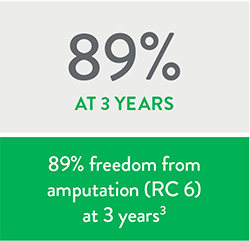
The Diamondback 360™ Peripheral Orbital Atherectomy System (OAS) has been well-studied in over 4,800 patients and in more than 7,000 lesions with durable results.1 In the LIBERTY 360 OAS sub-analysis, the primary patency was 89.7% at 2 years in RC2-3 patients2 and the 3-year freedom from major amputation was 89% in the RC6 patients.3
Proven Safety Record:


Diamondback 360 Peripheral OAS Clinical Studies
LIBERTY 360º
LIBERTY is the largest, contemporary, real-world study to evaluate procedural and long-term clinical outcomes of endovascular device interventions in patients with symptomatic lower extremity Peripheral Artery Disease (PAD).5
Study Design4,5
- Prospective, observational, multicenter study that includes any FDA-cleared or approved technology to treat claudication and Critical Limb Ischemia (CLI).
- 1,204 patients enrolled at 51 sites and were followed for up to five years. Four core labs were used for independent analysis.
- Key endpoints: Procedural and lesion success, Major Adverse Events (MAEs), Duplex Ultrasound (DUS), Quality of Life (QoL), and Six-Minute Walk Test (6MWT).
- Key inclusion criteria: Rutherford classification (RC) 2 to 6, target lesion located within or extending into 10 cm above the medial epicondyle to the digital arteries (distal 1/3 of the SFA and below), and at least one lesion that can be treated with an endovascular device.

Conclusion
Peripheral Vascular Intervention (PVI) may be a reasonable treatment option across all Rutherford classes with durable results lasting to 3 years.5
- High freedom from major amputation at 3 years across all Rutherford classes.5
- In the OAS sub-analysis, freedom from major amputation rates at 3 years were even higher than in the full patient cohort.5
- Improved Quality of Life by 30 days and maintained to 3 years across all Rutherford classes.5
- High long-term patency rates through 2 years in RC2-3.6
- Improvement in mean number of wounds from 30 days to 2 years in RC4-6.6
CALCIUM 360º
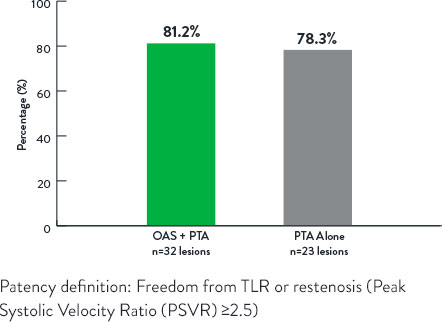
A prospective, randomized, multi-center study that compared the acute and long-term results of Orbital Atherectomy System (OAS) + Percutaneous Transluminal Angioplasty (PTA) versus PTA alone in calcified below-the-knee (BTK) lesions.7
Key Takeaways7
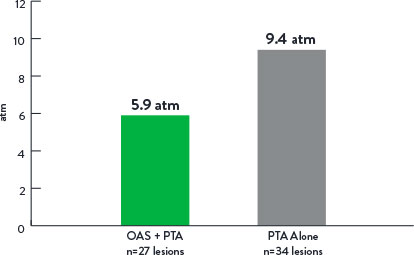
- Lower mean maximum balloon inflation pressure in OAS+PTA arm
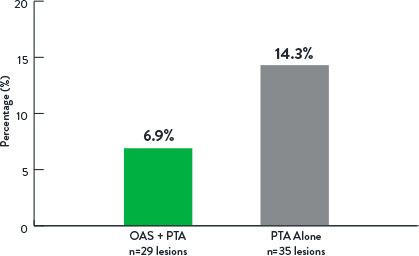
- Lower rate of major adverse events at 12-months in OAS+PTA arm
Study Design7
- 50 Critical Limb Ischemia (CLI) patients enrolled at 8 U.S. sites.
- Patients randomized to either OAS+PTA (25 pts) or PTA alone (25 pts) and followed for 12 months.
Patient Population7
| Demographics | OAS + PTA (n=25) | PTA Alone (n=25) | p-value |
|---|---|---|---|
| Mean Age | 70.7 ± 13.4 | 71.8 ± 10.9 | 0.75 |
| Male/Female | 68%/32% | 60%/40% | 0.77 |
| Diabetic Type 1 | 4% | 0% | 1.00 |
| Diabetic Type 2 | 68% | 56% | 0.56 |
| Renal Insufficiency (GFR <90 mL/min/1.73 m²) | 25% | 24% | 1.00 |
| Smoker (current or previous) | 60% | 60% | 1.00 |
| CAD | 44% | 56% | 0.57 |
| Hypertension | 84% | 84% | 1.00 |
| Dyslipidemia | 83% | 72% | 0.50 |
Study Results7
Mean Maximum Balloon Inflation Pressure (atm)
p=0.001
Rate of Bail-Out Stenting
p=0.44
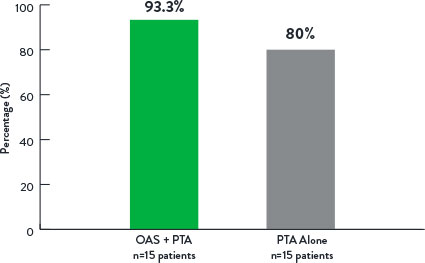
Freedom From Revascularization at 12 Months
p=0.14
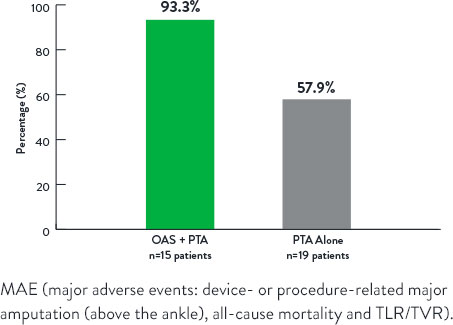
Freedom From Major Adverse Events at 12 Months
p=0.006
TRUTH
A prospective, single-arm, single-center study using Intravascular Ultrasound (IVUS) to assess Orbital Atherectomy System (OAS)-related plaque modification of femoropopliteal lesions.8
Key Takeaways8
- OAS modified the calcified component of the plaque burden (IVUS analysis).
- Calcium reduction was responsible for 86% of lumen area increase (IVUS analysis).
- Increase in minimum lumen area post-OAS (IVUS analysis).
- Low mean maximum balloon inflation pressure post-OAS of 5.2 atm.
Study Design8
- 25 patients enrolled at a single center.
- Patients treated with OAS+Percutaneous Transluminal Angioplasty (PTA) and followed for 12 months.
- IVUS images collected pre-OAS, post-OAS, and post-PTA (IVUS Core Lab).
Patient Population8
| Baseline Characteristics | (n=25) |
|---|---|
| Age | 70.4 ± 7.8 years |
| Gender (Male) | 19/25 (76.0%) |
| eGFR (mL/min/1.73 m2) | 70.9 ± 25.0 |
| History of diabetes (Type I or II) | 18/25 (72.0%) |
| History of hyperlipidemia | 25/25 (100.0%) |
| History of hypertension | 25/25 (100.0%) |
| Smoker (current or former) | 21/25 (84.0%) |
| Rutherford classification | 3.0 ± 0.0 |
Study Results8
- The mean maximum balloon inflation pressure was 5.2±1.2 atm.
- Virtual histology IVUS (VH-IVUS) analysis revealed at the maximum calcium ablation site that calcium reduction was responsible for 86% of the lumen area increase.
- The minimum lumen area increased from 4.0 mm² to 9.1 mm² (P<0.0001).
| Baseline (n=25) | 12-Month Follow-up (n-22) | p-value | |
|---|---|---|---|
| Rutherford Classification (RC) | <0.001 | ||
| Asymptomatic (RC 0) | 0 (0.0%) | 13 (59.1%) | |
| Mild Claudication (RC 1) | 0 (0.0%) | 8 (36.4%) | |
| Moderate Claudication (RC 2) | 0 (0.0%) | 1 (4.5%) | |
| Severe Claudication (RC 3) | 25 (100.0%) | 0 (0.0%) | |
| ABI* | 0.74 ± 0.13 (n=22) | 0.95 ± 0.15 (n=21) | <0.001 |
*Greater of posterior tibial or dorsalis pedis systolic pressure divided by maximum of left or right brachial systolic pressure.
At 12 months, the freedom from target lesion revascularization (FF TLR) rate was 91.8%, and ankle-brachial index and Rutherford classification improved significantly from baseline through follow-up.
COMPLIANCE 360°
A prospective, randomized, multi-center study that compared the acute and long-term results of Orbital Atherectomy System (OAS) + Percutaneous Transluminal Angioplasty (PTA) versus PTA alone in calcified above-the-knee (ATK) lesions.9
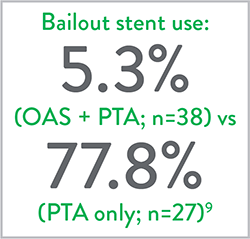
Key Takeaways9
- Lower mean maximum balloon inflation pressure in OAS+PTA arm.
- Lower adjunctive stent rate in OAS+PTA arm.
- OAS demonstrated reduced stenting with durable results out to 12 months versus PTA alone.
Study Design9
- 50 patients enrolled at 9 U.S. sites.
- Patients randomized to either OAS+PTA (25 pts) or PTA alone (25 pts) and followed for 12 months.
Patient Population9
| Comorbidity | OAS + PTA (n=25) | PTA Alone (n=25) | p-value |
|---|---|---|---|
| Mean Age ± SD | 68.0 ± 11.0 | 71.3 ± 10.5 | 0.27 |
| Female gender, n (%) | 18 (72) | 16 (64) | 0.76 |
| Non-Caucasian, n (%) | 9 (36) | 4 (16) | 0.19 |
| Coronary artery disease, n (%) | 12 (48) | 16 (64) | 0.39 |
| Diabetes, n (%) | 18 (72) | 10 (40) | 0.05 |
| Hypertension, n (%) | 22 (88) | 18 (72) | 0.29 |
| Hyperlipidemia, n (%) | 23 (92) | 21 (84) | 0.67 |
| Smoker (current or former), n (%) | 22 (88) | 22 (88) | >0.99 |
Study Results9
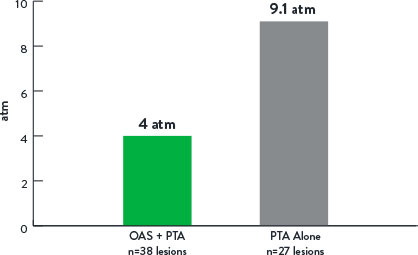
Mean Maximum Balloon Inflation Pressure (atm)
p<0.001
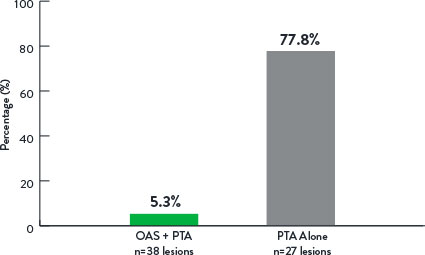
Adjunctive Stenting
p<0.001
Patency at 12 Months
p<0.99
REACH PVI
REACH PVI is a prospective, observational, single-arm, multi-center, post-market clinical study designed to prospectively evaluate acute clinical outcomes of Orbital Atherectomy (OA) via Transradial Access (TRA) for treatment of Peripheral Artery Disease (PAD) in lower extremity lesions.10
Key Takeaways10
- Average lesion length was 98.3 ± 87.5 mm.
- 78.0% of the lesions were severely calcified.
- Treatment success was 98.0%; in only one case, the result was sub-optimal (>30% stenosis with stent placement), and a significant dissection was reported.
- No serious distal embolization, serious thrombus formation, or serious acute vessel closure were observed intra- or post-procedurally.

Study Design10
- Six (6) sites enrolled 50 subjects in the REACH PVI study that were followed post-procedure through the first standard of care follow-up visit (7 to 45 days from the index procedure).
- The primary outcomes was defined as the successful completion of OA treatment of the target lesion via transradial access without serious transradial access-related events.
- The secondary outcome was treatment success, defined as residual stenosis less than 50% without stent implantation or less than 30% with stent implantation, and without any significant angiographic complications.
Study Results10
- The average lesion length was 98.3 ± 87.5 mm and 78.0% of the lesions were severely calcified.
- Balloon angioplasty was performed in 98.0% of target lesions, while a stent was deployed in 16.0%.
- Treatment success was 98.0%; in only one case the result was sub-optimal (>30% stenosis with stent placement) and a significant dissection was reported.
- No serious distal embolization, serious thrombus formation or serious acute vessel closure were observed intra- or post-procedurally.
Patient Population10
| Variables | (n=50) |
|---|---|
| Mean Age | 70.9 ± 10.3 |
| Male | 36 (72%) |
| Diabetes | 27 (54%) |
| Coronary Artery Disease | 32 (64%) |
| Current Smoker | 15 (30%) |
| Previous Smoker | 28 (56%) |
| Hypertension | 44 (88%) |
| Moderate Claudication (RC 2) | 6 (12%) |
| Severe Claudication (RC 3) | 31 (62%) |
| Ischemic Rest Pain (RC 4) | 12 (24%) |
| Minor Tissue Loss (RC 5) | 1 (2%) |
References
- Data on file at Abbott. Count updated on Jan 6, 2020—subject to change (include PAD I, PAD II, OASIS, CONFIRM, CALCIUM, COMPLIANCE, TRUTH, CLARITY, LIBERTY, OPTIMIZE, REACH PVI).
- Adams, GL, Outcomes of Orbital Atherectomy Treatment in Symptomatic Peripheral Artery Disease Patients: Sub-analys of the LIBERTY 360 Study. J Vasc Surg. 2019;70(5 Suppl):E188-E189.
- Giannopoulos S, et al. Three-Year Outcomes of Orbital Atherectomy for the Endovascular Treatment of Infrainguinal Claudication or Chronic Limb-Threatening Ischemia. J Endovasc Ther. 2020;27(5):714-725.
- Adams GL, et al. The LIBERTY Study: Design of a Prospective, Observational, Multicenter Trial to Evaluate the Acute and Long-Term Clinical and Economic Outcomes of Real-World Endovascular Device Interventions in treating Peripheral Artery Disease. Am Heart J. 2016;174:14-21.
- Mustapha JA. LIBERTY 360 Trial 3-Year Update. Presented at AMP 2019.
- Mustapha JA. Late Breaking: LIBERTY 360 Trial 2-Year Update. Presented at AMP 2018.
- Shammas NW, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomized pilot trial. J Endovasc Ther. 2012;19(4):480-8.
- Babaev A, et al. Orbital Atherectomy Plaque Modification Assessment of the Femoropopliteal Artery Via Intravascular Ultrasound (TRUTH Study). Vasc Endovascular Surg. 2015;49(7):188-94.
- Dattilo R, et al. The COMPLIANCE 360° Trial: a randomized, prospective, multicenter, pilot study comparing acute and long-term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. J Invasive Cardiol. 2014;26(8):355-60.
- Lodha A, et al. Transradial endovascular intervention: Results from the radial access for navigation to your chosen lesion for peripheral vascular intervention (REACH PVI) study. Cardiovasc Revasc Med. 2022;36:115-120.
MAT-2314266 v2.0
Important Safety Information
Diamondback 360™ Peripheral Orbital Atherectomy System, Diamondback 360™ Peripheral Orbital Atherectomy System – Exchangeable Series

Applies to Diamondback 360™ Peripheral Orbital Atherectomy System only: Including the Orbital Atherectomy Device (OAD) with GlideAssist™, Saline Pump, Viperwire Advance™ Peripheral Guide Wire, and Viperwire Advance™ with Flex Tip Peripheral Guide Wire
Applies to Diamondback 360™ Peripheral Orbital Atherectomy System – Exchangeable Series only: Including the Orbital Atherectomy Device (OAD), Handle, Orbital Atherectomy Cartridge, Saline Pump, Viperwire Advance™ Peripheral Guide Wire, and Viperwire Advance™ with Flex Tip Peripheral Guide Wire
INDICATIONS
The Diamondback 360™ Peripheral Orbital Atherectomy System/Diamondback 360™ Peripheral Orbital Atherectomy System – Exchangeable Series (OAS) is a percutaneous orbital atherectomy system indicated for use as therapy in patients with occlusive atherosclerotic disease in peripheral arteries and who are acceptable candidates for percutaneous transluminal atherectomy.
The OAS Solid, Classic and Micro Crowns support removal of stenotic material from artificial arteriovenous dialysis fistulae (AV shunt). The system is a percutaneous orbital atherectomy system indicated as a therapy in patients with occluded hemodialysis grafts who are acceptable candidates for percutaneous transluminal angioplasty.*
*The 2.00 Max Crown has not been tested to support removal of stenotic material from artificial dialysis fistulae (AV shunt).
CONTRAINDICATIONS
Use of the OAS is contraindicated in the following situations:
- The guide wire cannot be passed across the peripheral lesion.
- The system cannot be used in coronary arteries.
- The target lesion is within a bypass graft or stent.
- The patient has angiographic evidence of thrombus; thrombolytic therapy must be instituted prior to atherectomy.
- The patient has angiographic evidence of significant dissection at the treatment site. The patient may be treated conservatively to permit the dissection to heal before treating the lesion with the OAS.
WARNINGS
- Do not use the OAD in a vessel that is too small for the crown. The reference vessel diameter at the treatment area must be at least 2.00 mm in diameter for the 1.25mm Micro crown.
- If mechanical failure of the OAD occurs before or during the atherectomy procedure, discontinue use immediately. Do not attempt to use a damaged OAD or other system component. Use of damaged components may result in system malfunction or patient injury.
- Do not use the OAD during spasm of the vessel.
- Only use approved and compatible Viperwire Advance™ guide wires. Reference the Instructions for Use for the appropriate guide wire to use based on the OAD shaft configuration. Follow CSI’s instructions related to guide wire use.
- Do not continue treatment if the guide wire or the OAD becomes sub-intimal.
- Immediately stop use if the OAD stalls. Review for complications and mechanical failure if a stall condition occurs. Do not change to a higher speed if the OAD stalls.
- Performing treatment in vessels or bifurcations that are excessively tortuous or angulated may result in vessel damage.
- Always use fluoroscopy when advancing the guide wire to avoid misplacement, dissection, or perforation. The OAD tracks over the guide wire, so it is imperative that the guide wire be initially placed through the stenotic lumen and not in a false channel.
- Do not inject contrast while OAD crown is spinning. OAD failure or patient harm may occur.
- Do not attempt aspiration through the OAD or saline line while placed within the body. If saline is pulled out through the OAD or saline line, air may enter the system.
- If air is noticed in the system while the OAD is within the body, discontinue treatment by pressing the OAS Pump power button and carefully remove the OAD driveshaft and crown from the introducer sheath/guide catheter.
- Handle the OAD and guide wire carefully. A tight loop, kink, or bend in the guide wire may cause damage and system malfunction during use.
- Never operate the OAD without normal saline and lubricant solution. Flowing saline and lubricant solution is required for cooling and lubricating the OAD during use to avoid overheating and permanent damage to the OAD and possible patient injury.
- The crown at the distal tip of the OAD operates at very high speeds. Do not allow body parts or clothing to come into contact with the crown. Physical injury or entanglement may occur.
- Never advance the orbiting crown to the point of contact with the guide wire spring tip. Distal detachment and embolization of the tip may result.
- Always advance the orbiting, abrasive crown by using the crown advancer knob. Never advance the orbiting crown by advancing the shaft or handle. Guide wire buckling may occur, and perforation or vascular trauma may result.
- Always keep the crown advancing or retracting while it is at high rotational speeds. Do not allow the crown to remain in one location for more than 2–3 seconds. Maintaining the crown in one location while it is orbiting at high speeds may lead to excessive tissue removal.
- Do not start or stop orbiting of the crown when tight in a lesion.
- Never force the crown when rotational or translational resistance occurs; vessel perforation may occur. If resistance to motion is noted, retract the crown and stop treatment immediately. Use fluoroscopy to analyze the situation.
- When treating tight stenosis, create a channel at low or medium speed before traversing the lesion at high speed. Crossing a tight stenosis on high speed may cause the shaft and/or guide wire to fracture as a result of excessive force.
- While advancing the crown through the introducer sheath/guide catheter, do not activate crown rotation. The crown must not spin while located within the introducer sheath/guide catheter.
- Do not prolapse or bend the guide wire core. If the spring tip becomes prolapsed, keep the bend/prolapse contained within the spring tip section only. Spinning over a prolapsed or bent guide wire core can result in damage to the guide wire or OAD.
- The system should not be used on children or pregnant women.
- Do not re-use or re-sterilize the OAD. If the OAD is re-used, the OAD may not function as intended and serious infection, leading to potential harm and/or death, may occur.
- Do not spin the crown in GlideAssist™ mode with the guide wire brake lever in the unlocked position, without first securing the guide wire by holding it with fingers or with the guide wire torquer. If using the guide wire torquer, ensure that it is securely fastened to the guide wire before starting to spin the crown. Failure to secure the guide wire when the brake is unlocked could allow the guide wire to spin and/or migrate while in GlideAssist™ mode which may result in patient harm.
PRECAUTIONS
- Do not use the product if the sterile packaging appears damaged or the shelf life has expired.
- User should take precautions when using the OAS or its components in conjunction with other medical equipment
- Do not flip contents of trays into the sterile field as damage may occur. Components within trays must be carefully removed and placed into sterile field to avoid damage.
- Follow standard hospital atherectomy policies and procedures, including those related to anticoagulation and vasodilator therapy.
- Radiographic equipment for fluoroscopy should be used to provide high-resolution images. Guide wires and catheters should only be manipulated under fluoroscopy.
- Because of the torque responsiveness of ViperWire™ peripheral guide wires , they are more difficult to handle than other commercially available guide wires used in peripheral angioplasty. Exercise care when using these guide wires.
- Use only normal saline and lubricant solution as the infusate. (Drugs such as vasodilators are added to the infusate at the physician’s discretion). The OAD may malfunction if contrast or other substances are injected into the OAD infusion port.
- Do not operate the OAD without using recommended ViperSlide™ Lubricant concentration (maximum speeds may not be achieved without lubricants).
- Ensure the OAD strain relief remains straight during atherectomy treatment.
- To relieve compression in the driveshaft, lock the crown advancer knob at 1 cm from the full back position, advance device over the wire to a position proximal from the lesion, deploy the guide wire brake, then unlock the crown advancer knob and move it fully proximal. If the OAD is started with existing compression in the driveshaft, it may result in the crown springing forward.
- If 1:1 motion is not observed between the crown advancer knob and the crown, retract and re-advance the crown into the lesion. Repeat retracting and advancing the crown into the lesion until 1:1 movement is observed. If the knob and the crown are not moving together, the crown may be driven into the lesion with too much force and may result in the crown springing forward on exiting the lesion.
- When moving the crown back and forth across the lesion, employ a series of intermittent treatment intervals and rest periods. 30-second rest periods are recommended after 30-second treatment intervals, with a maximum total treatment time of 8 minutes.
- Monitor the saline fluid level during the procedure. Normal saline and lubricant solution infusion is critical to OAD performance.
- Do not kink or crush the saline tubing. Flow of saline will be reduced.
- Check the saline tubing and connections for leaks during the procedure.
- Do not allow fluid to leak onto electrical connections of the OAS pump.
Applies to Diamondback 360™ Peripheral OAS – Exchangeable Series only:
- Do not detach the cartridge from the handle when the OAD is over the guide wire. Kinking of the guide wire and/or not being able to reconnect the cartridge and the handle may occur.
- Do not track only the cartridge into the patient and then attempt to connect the handle. Kinking of the guide wire and/or not being able to reconnect the cartridge and the handle may occur.
- Do not attempt to load a guide wire with a crossing profile >0.014” through the proximal end of the OAD. Guide wire with a crossing profile >0.014” will not fit through the internal components of the OAD.
- Do not attempt to remove the cartridge from the handle when spinning.
POTENTIAL ADVERSE EVENTS
Potential adverse events that may occur and/or require intervention include, but are not limited to:
- Allergic reaction to medication/media/device components
- Amputation
- Anemia
- Aneurysm
- Bleeding complications which may require transfusion
- Cerebrovascular accident (CVA)
- Death
- Distal embolization
- Entry site complications
- Hemolysis
- Hypotension/hypertension
- Infection
- Myocardial infarction
- Pain
- Pseudoaneurysm
- Restenosis of treated segment that may require revascularization
- Renal insufficiency/failure
- Slow flow or no reflow phenomenon
- Thrombus
- Vessel closure, abrupt
- Vessel injury, including dissection and perforation that may require surgical repair
- Vessel spasm
- Vessel occlusion
Diamondback 360™ Peripheral OAS/Diamondback 360™ Peripheral OAS– Exchangeable Series are manufactured and distributed by Cardiovascular Systems, Inc. (CSI). CSI is a subsidiary of the Abbott Group of Companies.
MAT-2303958 v1.0
