Indications
The Ultreon™ 2.0 Software is intended to be used only with compatible OPTIS™ Next Imaging Systems.
The OPTIS™ Next Imaging Systems with a compatible Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for the imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is intended for use in vessels 2.0 to 3.5 mm in diameter. The Dragonfly™ OPTIS™ Imaging Catheter or Dragonfly OpStar™ Imaging Catheter is not intended for use in the left main coronary artery or in a target vessel which has undergone a previous bypass procedure.
The OPTIS™ Next Imaging Systems are intended for use in the catheterization and related cardiovascular specialty laboratories and will further compute and display various physiological parameters based on the output from one or more electrodes, transducers, or measuring devices. The physician may use the acquired physiological parameters, along with knowledge of patient history, medical expertise, and clinical judgment to determine if therapeutic intervention is indicated.
Contraindications
Use of the Ultreon™ 2.0 Software is contraindicated where introduction of any catheter would constitute a threat to patient safety. Contraindications include:
- Bacteremia or sepsis
- Major coagulation system abnormalities
- Patients diagnosed with coronary artery spasm
- Patients disqualified for coronary artery bypass graft (CABG) surgery
- Patients disqualified for percutaneous transluminal coronary angioplasty (PTCA)
- Severe hemodynamic instability or shock
- Total occlusion
- Large thrombus
- Acute renal failure
- Inability to tolerate systemic anticoagulation is a contraindication to use of OCT for coronary imaging.
- The system has no patient alarm functions. Do not use for cardiac monitoring.
Complications
The following complications may occur as a consequence of intravascular imaging and catheterization procedure:
- Abnormal heart rhythm or arrhythmias
- Acute myocardial infarction
- Allergic reaction to the contrast media or drug administered for the procedure
- Arterial dissection, injury, or perforation
- Bleeding
- Catheter access site reactions: inflammation or granuloma
- Coronary artery spasm
- Death
- Embolism
- Hypotension
- Infection
- Myocardial ischemia
- Renal insufficiency or failure from contrast media use
- Repeat revascularization
- Thrombus formation, abrupt closure, or total occlusion
- Tissue necrosis
- Unstable angina
Warnings
- Prior to use, please review the Instructions for Use supplied with the Dragonfly™ Imaging Catheter for more information.
- Appropriate anticoagulant and vasodilator therapy must be used during the procedure as needed.
- Ensure that no air is introduced into the system during the Dragonfly™ Imaging Catheter insertion.
- Observe all advancement and movement of the Dragonfly™ Imaging Catheter under fluoroscopy. Always advance and withdraw the catheter slowly. Failure to observe device movement fluoroscopically may result in vessel injury or device damage. To ensure proper placement, do not move the guide wire after the Dragonfly™ Imaging Catheter is in place.
- If resistance is encountered during advancement or withdrawal of the Dragonfly™ Imaging Catheter, stop manipulation and evaluate under fluoroscopy. If the cause of resistance cannot be determined or mitigated, carefully remove the Dragonfly™ Imaging Catheter and guidewire together as a unit from the patient.
- Leave the guide wire engaged with the Dragonfly™ Imaging Catheter at all times during use. Do not withdraw or advance the guide wire prior to withdrawing the Dragonfly™ Imaging Catheter.
- The Dragonfly™ Imaging Catheter should never be forced into lumens that are narrower than the Dragonfly™ Imaging Catheter body or forced through a tight or heavily calcified lesion.
- The Dragonfly™ Imaging Catheter should not be advanced through abnormally tortuous anatomy.
- When advancing or retracting a Dragonfly™ Imaging Catheter with a monorail tip through a stented vessel, the Dragonfly™ Imaging Catheter may engage the stent between the junction of the Dragonfly™ Imaging Catheter and guide wire, resulting in entrapment of catheter / guide wire, catheter tip separation, stent dislocation, and / or vascular injury.
- Refer to the contrast media Instructions for Use for general warnings and precautions relating to use of contrast media.
- Before creating an OCT recording, review “Performing an OCT Procedure” for additional warnings and cautions in the IFU.
Precautions
- Safety and effectiveness have been established for the following patient population: adult patients undergoing non-emergent percutaneous coronary interventions in lesions with reference vessel diameters between 2.0 to 3.5 mm, which are not located in the left main coronary artery or in a target vessel which has undergone previous bypass procedures.
- Follow all instructions, warnings, and cautions provided in “Patient Safety” in the IFU.
- All operators must be knowledgeable in performing OCT and physiological procedures prior to using the Ultreon™ 2.0 Software, OPTIS™ Next Imaging System, and the Dragonfly™ Imaging Catheter.
- When using saline, heparinized saline is recommended.
- Monitor the OCT image for indications of Dragonfly™ Imaging Catheter optical failure. If optical failure is suspected, remove the Dragonfly™ Imaging Catheter from the patient, press “Unload” on the drive motor and optical controller (DOC), detach the catheter, and replace it with a new one.
- If the pullback triggers before contrast is injected, repeat the pullback.
- For optimal imaging, only use 100% contrast media.
- Use the minimum flush rate and volume required to image the desired anatomy.
- To obtain accurate measurements, be sure the selection for the Flush Medium is the same as the medium in which you are imaging.
- The Dragonfly™ Imaging Catheter must be purged prior to connection to the DOC to prevent damage to the imaging core.
- Do not insert or remove the Dragonfly™ Imaging Catheter while the DOC is scanning. Do not attempt to disconnect the catheter from the DOC while the “lock” LED is blinking as it could damage the catheter or the DOC. Refer to “Removing the Dragonfly™ Imaging Catheter” in the IFU.
- Never attempt to attach or detach a catheter to the DOC while the "lock" LED is lit.
- Take care in handling the Dragonfly™ Imaging Catheter to prevent breaking the fiber-optics within the catheter. Kinking and bending of the catheter can cause damage. While connecting, ensure the proximal catheter segment is straight and aligned with the DOC. Never attempt to connect and operate the catheter while the catheter remains coiled within the hoop.
- Do not kink, sharply bend, pinch, or crush the Dragonfly™ Imaging Catheter at any time.
- The Dragonfly™ Imaging Catheter has no user serviceable parts. Do not attempt to repair or alter any part of the catheter assembly as provided.
- If you want to make measurements on files that will be exported to standard formats, you must make the measurements BEFORE exporting the images. Using non-OCT software to measure standard format images will not produce accurate measurements.
- Do not use images that have been exported to JPEG or Compressed AVI formats for clinical decision making. These formats use compression methods that may degrade the image quality.
- Artifacts may result in misrepresentation of L-mode data, so L-mode is not recommended for quantification of clinical information.
- It is the user’s responsibility to confirm the lumen contours of all the frames within the reference segment, and to make adjustments if necessary. Red frames indicate low confidence in the detected contours.
- Deleted files cannot be restored. After files have been deleted, they can only be imported back to your system from your archived copies.
- Restoring factory default settings resets ALL user-entered configuration values except the date and time. This button should be used only under the direction of qualified service personnel.
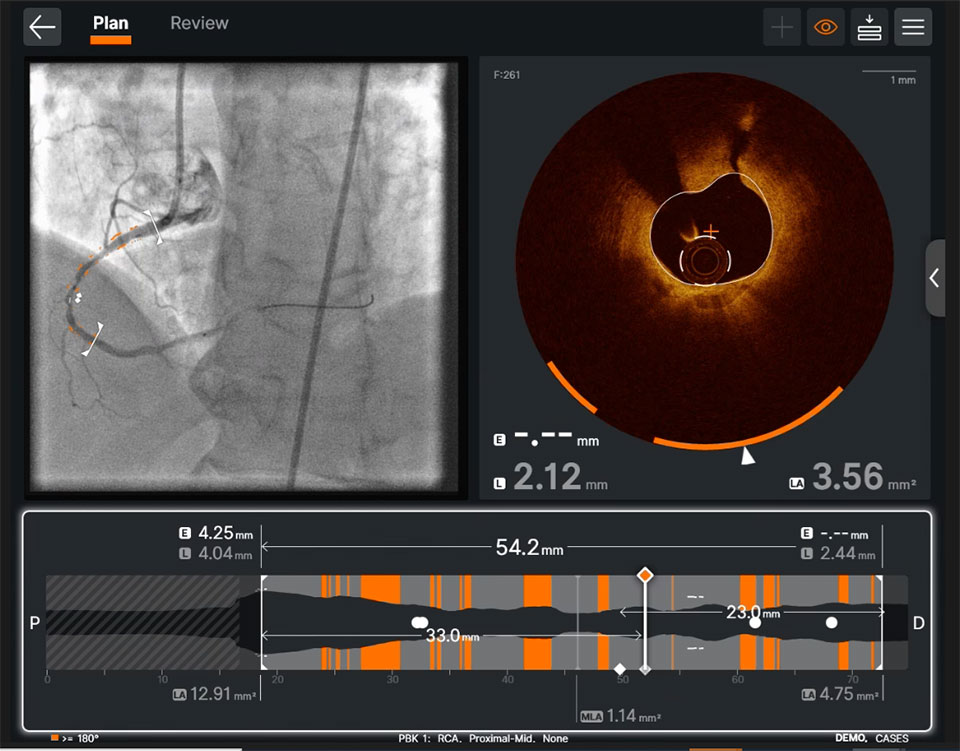
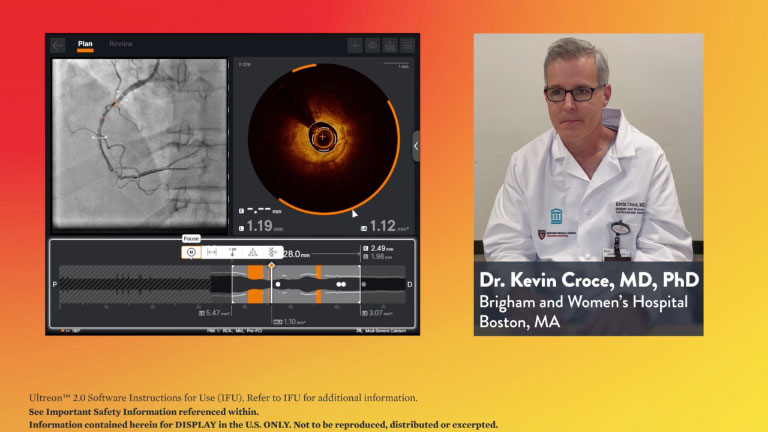
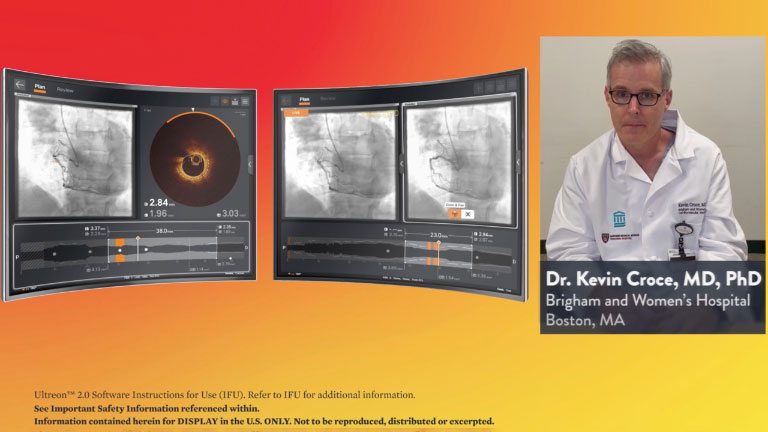
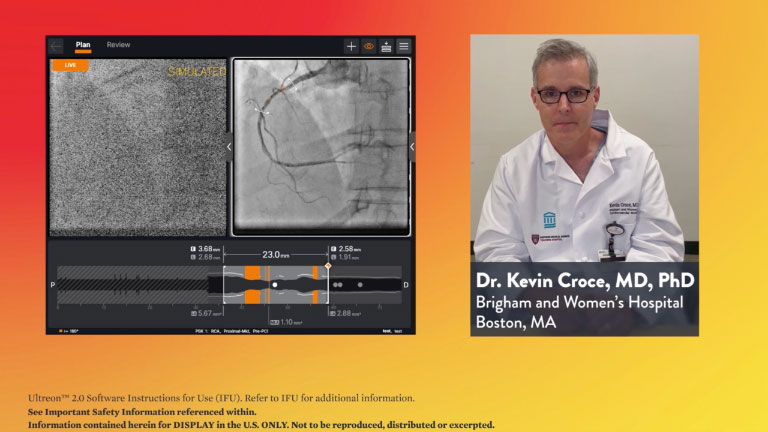





Stay Connected