Designed With Intention
Workhorse Guide Wires — Guide Wires you can rely on to handle a wide variety of everyday needs.
Easy To Shape | Atraumatic Tip to Minimize Perforation Risk1
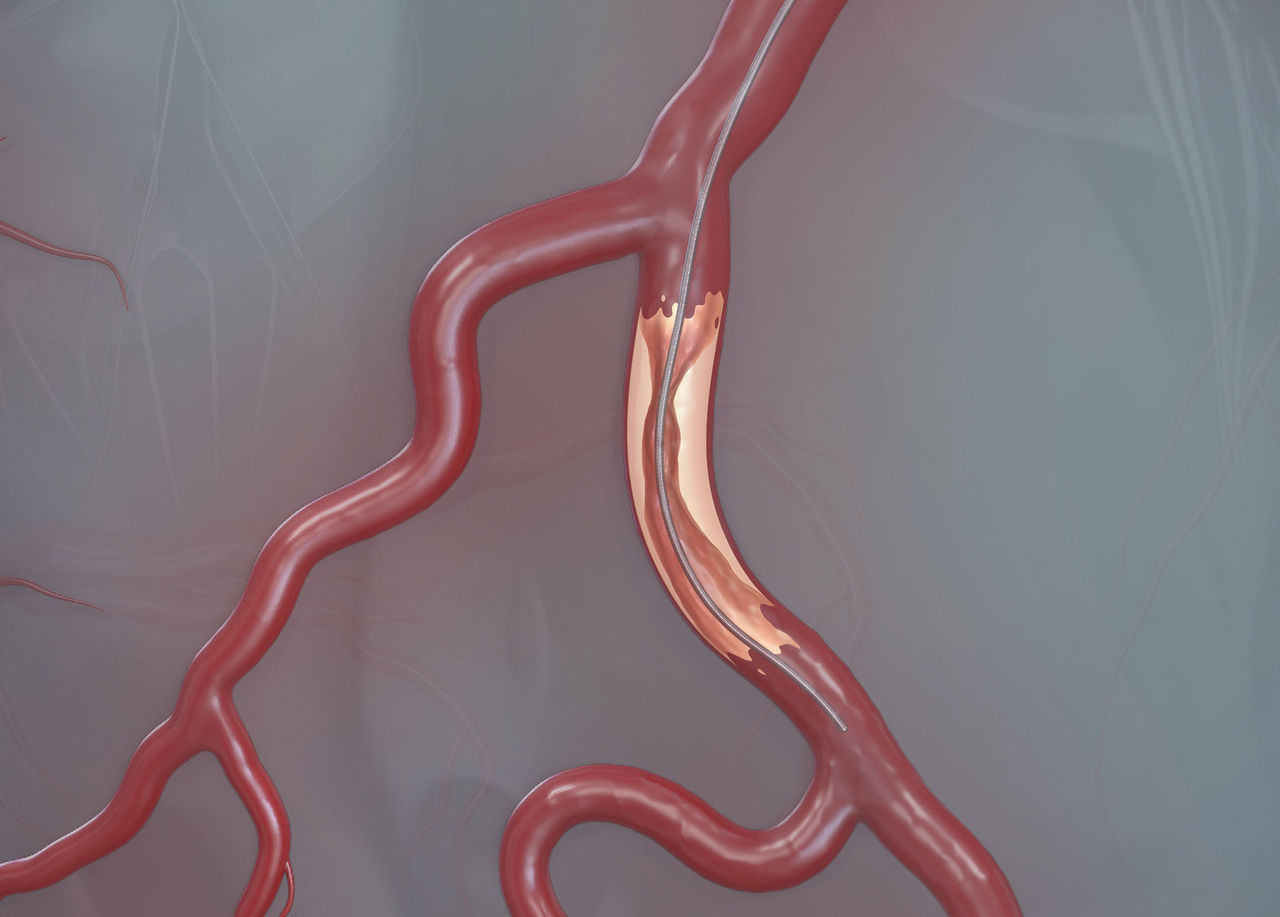
Tortuous Anatomy | Angulated/Bifurcated Vessels | Calcified Lesions
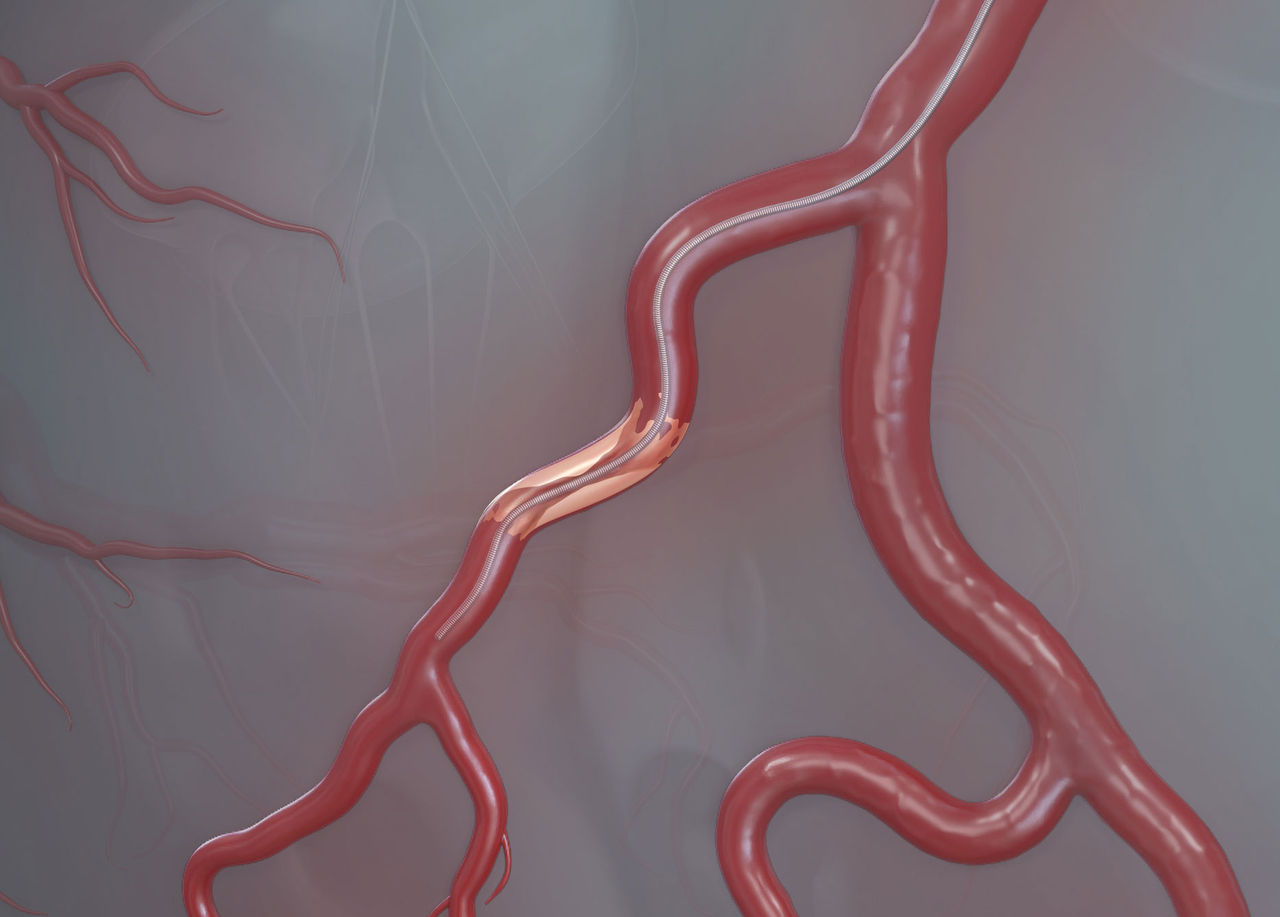
*Optional for HI-TORQUE BALANCE MIDDLWEIGHT UNIVERSAL™ Guide Wire and HI-TORQUE BALANCE MIDDLEWEIGHT UNIVERSAL II™ Guide Wire
Data on file at Abbott unless otherwise noted.
References
- Brilakis, Emmanouil. Manual of Percutaneous Coronary Interventions. October 2020.
MAT-2308285 v1.0
HI-TORQUE™ Guide Wires

INTENDED USE
All HI-TORQUE™ Guide Wires are intended to facilitate the placement of balloon dilatation catheters during percutaneous transluminal coronary angioplasty (PTCA) and percutaneous transluminal angioplasty (PTA).
INDICATIONS
Refer to the device label for any additional product-specific indications that may apply.
CONTRAINDICATIONS
HI-TORQUE™ Guide Wires Wires are not intended for use in the cerebral vasculature. Refer to the device label for any additional product-specific contraindications that may apply.
WARNINGS
This device is designed and intended for ONE-TIME USE ONLY. Do not resterilize and / or reuse.
Carefully observe the instructions under “Do Not” and “Do” below. Failure to do so may result in vessel trauma, guide wire damage, guide wire tip separation, or stent damage. If resistance is observed at any time, determine the cause under fluoroscopy and take remedial action as needed. Use the most suitable guide wire for the lesion being treated.
Do Not:
- Push, auger, withdraw, or torque a guide wire that meets resistance.
- Torque a guide wire if the tip becomes entrapped within the vasculature.
- Allow the guide wire tip to remain in a prolapsed condition.
Do:
- Advance or withdraw the guide wire slowly.
- Use the radiopaque marker of the interventional device to confirm position.
- Examine the tip movement under fluoroscopy before manipulating, moving, or torquing the guide wire.
- Observe the wire under fluoroscopy for tip buckling, which is a sign of resistance.
- Maintain continuous flush while removing and reinserting the guide wire to prevent air from entering the catheter system. Perform exchanges slowly to prevent air entry and / or trauma.
- When reintroducing the guide wire, confirm that the interventional device tip is free within the vessel lumen and that the tip is parallel to the vessel wall.
- Use extreme caution when moving a guide wire through a non-endothelialized stent, or through stent struts, into a bifurcated vessel. Use of this technique involves additional patient risks, including the risk that the wire may become caught on the stent strut.
- Consider that if a secondary wire is placed in a bifurcation branch, this wire may need to be retracted prior to stent deployment because there is additional risk that the secondary wire may become entrapped between the vessel wall and the stent.
PRECAUTIONS
Guide wires are delicate instruments and should be handled carefully. Prior to use and when possible during the procedure, inspect the guide wire carefully for bends, kinks, or other damage. Do not use damaged guide wires. Using a damaged guide wire may result in vessel damage and / or inaccurate torque response.
Confirm the compatibility of the guide wire diameter with the interventional device before actual use.
Free movement of the guide wire within the interventional device is an important feature of a steerable guide wire system, because it gives the user valuable tactile information. Test the system for any resistance prior to use. Adjust or replace the hemostatic valve with an adjustable valve if it is found to inhibit guide wire movement.
Never attach the torque device to the modified portion of the proximal end of the extendable guide wire; otherwise, guide wire damage may occur, preventing the ability to attach the DOC™ Guide Wire Extension.
HI-TORQUE™ Guide Wires with Hydrophilic Coating: Avoid abrasion of the hydrophilic coating.
Do not withdraw or manipulate the hydrophilic-coated wire through a metal cannula or sharp-edged object.
ADVERSE EVENTS
Potential Adverse Events associated with use of this device may include the following but not limited to perforation, dissection, occlusion, myocardial infarction, embolism and infection.
MAT-2213672 v1.0
HI-TORQUE TURNTRAC™
Guide Wire

INDICATIONS FOR USE
This HI-TORQUETM Guide Wire is intended to facilitate the delivery of catheter-based interventional devices during the following procedures:
• Percutaneous transluminal angioplasty (PTA)
• Percutaneous transluminal coronary angioplasty (PTCA)
This guide wire may also be used with compatible stent devices.
This device is designed and intended for ONE-TIME USE ONLY. Do not resterilize and / or reuse.
CONTRAINDICATIONS
Not intended for use in the cerebral vasculature.
WARNINGS
Not intended for use with atherectomy devices.
Carefully observe the instructions under “Do Not” and “Do” below. Failure to do so may result in vessel trauma, guide wire damage, guide wire tip separation, or stent damage. If resistance is observed at any time, determine the cause under fluoroscopy and take remedial action as needed. Use the most suitable guide wire for the lesion being treated.
Do Not:
- Push, auger, withdraw, or torque a guide wire that meets resistance.
- Torque a guide wire if the tip becomes entrapped within the vasculature.
- Allow the guide wire tip to remain in a prolapsed condition.
- Deploy a stent such that it will entrap the wire between the vessel wall and the stent.
Do:
- Advance or withdraw the guide wire slowly.
- Use the radiopaque marker of the interventional device to confirm position.
- Examine the tip movement under fluoroscopy before manipulating, moving, or torquing the guide wire.
- Observe the wire under fluoroscopy for tip buckling, which is a sign of resistance.
- Maintain continuous flush while removing and reinserting the guide wire to prevent air from entering the catheter system. Perform exchanges slowly to prevent air entry and / or trauma.
- When reintroducing the guide wire, confirm that the interventional device tip is free within the vessel lumen and that the tip is parallel to the vessel wall.
- Use extreme caution when moving a guide wire through a non-endothelialized stent, or through stent struts, into a bifurcated vessel. Use of this technique involves additional patient risks, including the risk that the wire may become caught on the stent strut.
PRECAUTIONS
Guide wires are delicate instruments and should be handled carefully. Prior to use and when possible during the procedure, inspect the guide wire carefully for bends, kinks, or other damage. Do not use damaged guide wires.
Using a damaged guide wire may result in vessel damage and / or inaccurate torque response.
This device should be used only by physicians trained in angiography and percutaneous transluminal coronary angioplasty (PTCA), and / or percutaneous transluminal angioplasty (PTA).
Confirm the compatibility of the guide wire diameter with the interventional device before actual use.
Free movement of the guide wire within the interventional device is an important feature of a steerable guide wire system, because it gives the user valuable tactile information. Test the system for any resistance prior to use. Adjust or replace the hemostatic valve with an adjustable valve if it is found to inhibit guide wire movement.
Never attach the torque device to the modified portion of the proximal end of the extendable guide wire; otherwise, guide wire damage may occur, preventing the ability to attach the DOCTM Guide Wire Extension.
HI-TORQUETM Guide Wires with Hydrophilic Coating: Avoid abrasion of the hydrophilic coating. Do not withdraw or manipulate the hydrophilic-coated wire through a metal cannula or sharp-edged object.
ADVERSE EVENTS
Potential adverse events associated with use ofthis device may include the following but not limited to:
- Perforation
- Dissection
- Occlusion
- Myocardial infarction
- Embolism
- Infection
- Allergic reaction or hypersensitivity to latex, contrast agent, anesthesia, device materials, and drug reactions to anticoagulation, or antiplatelet drugs
- Vasoconstriction
- Vasospasm
- Hypotension
- Hypertension
MAT-2104727 v2.0
HI-TORQUE VERSATURN™ Guide Wire

INDICATIONS FOR USE
This HI-TORQUE™ Guide Wire is intended to facilitate the delivery of catheter-based interventional devices during the following procedures:
- Percutaneous transluminal angioplasty (PTA)
- Percutaneous transluminal coronary angioplasty (PTCA)
This guide wire may also be used with compatible stent devices.
This device is designed and intended for ONE-TIME USE ONLY. Do not resterilize and / or reuse.
CONTRAINDICATIONS
Not intended for use in the cerebral vasculature.
WARNINGS
Not intended for use with atherectomy devices.
Carefully observe the instructions under “Do Not” and “Do” below. Failure to do so may result in vessel trauma, guide wire damage, guide wire tip separation, or stent damage. If resistance is observed at any time, determine the cause under fluoroscopy and take remedial action as needed. Use the most suitable guide wire for the lesion being treated.
Do Not:
- Push, auger, withdraw, or torque a guide wire that meets resistance.
- Torque a guide wire if the tip becomes entrapped within the vasculature.
- Allow the guide wire tip to remain in a prolapsed condition.
- Deploy a stent such that it will entrap the wire between the vessel wall and the stent.
Do:
- Advance or withdraw the guide wire slowly.
- Use the radiopaque marker of the interventional device to confirm position.
- Examine the tip movement under fluoroscopy before manipulating, moving, or torquing the guide wire.
- Observe the wire under fluoroscopy for tip buckling, which is a sign of resistance.
- Maintain continuous flush while removing and reinserting the guide wire to prevent air from entering the catheter system. Perform exchanges slowly to prevent air entry and / or trauma.
- When reintroducing the guide wire, confirm that the interventional device tip is free within the vessel lumen and that the tip is parallel to the vessel wall.
- Use extreme caution when moving a guide wire through a non-endothelialized stent, or through stent struts, into a bifurcated vessel. Use of this technique involves additional patient risks, including the risk that the wire may become caught on the stent strut.
PRECAUTIONS
Guide wires are delicate instruments and should be handled carefully. Prior to use and when possible during the procedure, inspect the guide wire carefully for bends, kinks, or other damage. Do not use damaged guide wires. Using a damaged guide wire may result in vessel damage and / or inaccurate torque response.
Confirm the compatibility of the guide wire diameter with the interventional device before actual use.
Free movement of the guide wire within the interventional device is an important feature of a steerable guide wire system, because it gives the user valuable tactile information. Test the system for any resistance prior to use. Adjust or replace the hemostatic valve with an adjustable valve if it is found to inhibit guide wire movement.
Never attach the torque device to the modified portion of the proximal end of the extendible guide wire; otherwise, guide wire damage may occur, preventing the ability to attach the DOC™ Guide Wire Extension.
HI-TORQUE™ Guide Wires with Hydrophilic Coating: Avoid abrasion of the hydrophilic coating. Do not withdraw or manipulate the hydrophilic-coated wire through a metal cannula or sharp-edged object.
ADVERSE EVENTS
Potential adverse events associated with use of this device may include the following but not limited to perforation, dissection, occlusion, myocardial infarction, embolism and infection.
MAT-2104726 v2.0

Stay Connected