True Controlled Compliance, High Pressure Tolerance and Re-crossability
- 2.0 – 5.0 mm diameter range with balloon lengths up to 26 mm for broad range of vascular treatment
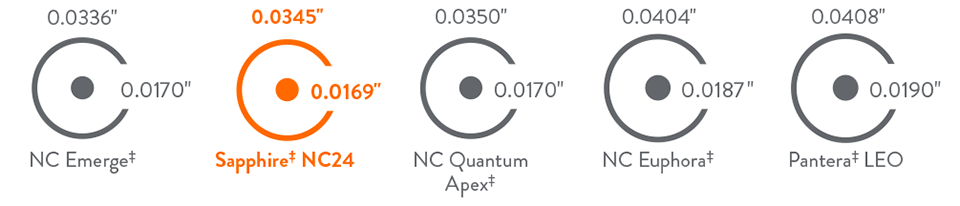
- Competitive crossability and tip entry profile
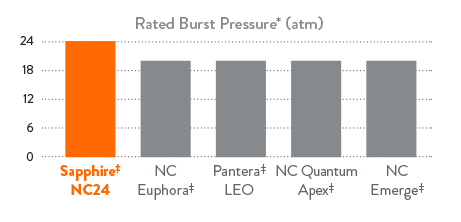
- Highest rated burst pressure (RBP) at 24 atm
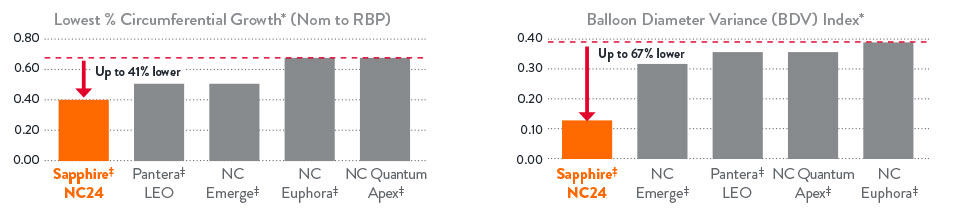
- Controlled balloon compliance for most accurate sizing, vessel optimization and minimal dog-bone effect
Competitive Crossability and Tip Entry Profile
Tip Entry and Crossing Profile Comparison (Ø3.0 mm)
Highest RBP at 24 atm
Controlled Balloon Compliance for Most Accurate Sizing, Vessel Optimization and Minimal Dog-Bone Effect
Technical Information
| Sapphire‡ NC24 Coronary Dilatation Catheter | |
|---|---|
| Platform | Rapid Exchange (RX) |
| Compliance | Non-compliant |
| Nominal Pressure | 14 atm |
| Rated Burst Pressure | 24 atm (Ø2.0 - 3.5 mm); 22 atm (Ø3.75 - 4.0 mm); 20 atm (Ø4.5 - 5.0 mm) |
| Crossing Profile (Ø3.0 mm) | 0.0345 in |
| Proximal Shaft | 2.1 F |
| Distal Shaft | 2.36 F - 2.7 F (Ø2.0 - 5.0 mm) |
| Tip Length | 2.0 mm (Ø2.0 - 3.0 mm); 2.5 mm (Ø3.25 - 5.0 mm) |
| Balloon Lengths (mm) | 8, 10, 12, 15, 18, 22, 26 |
| Balloon Diameters (mm) | 2.0, 2.25, 2.5, 2.75, 3.0 3.25, 3.50, 3.75, 4.0, 4.5, 5.0 |
| Balloon Material | Nylon and Pebax (dual layer) |
| Balloon Folds | 3 folds (Ø2.0 - 4.0 mm); 5 folds (Ø4.5 - 5.0 mm) |
| Marker Bands | 2 markers (Ø2.0 - 5.0 mm) |
| Catheter Length | 140 cm |
| Coating | Hydrophilic (tip to guidewire exit port); Hydrophobic (guidewire lumen) |
| Guide Wire Compatibility | 0.014 in |
| Guide Catheter Compatibility | 5 F (Ø2.0 - 4.0 mm); 6 F (Ø4.5 - 5.0 mm) |
| Not Made With Natural Rubber Latex | Yes |
Ordering Information
| Balloon Diameter (mm) | Balloon Working Length (mm) | ||||||
|---|---|---|---|---|---|---|---|
| 8 | 10 | 12 | 15 | 18 | 22 | 26 | |
| 2.0 | 492000812 | 492001012 | 492001212 | 492001512 | 492001812 | 492002212 | 492002612 |
| 2.25 | 492250812 | 492251012 | 492251212 | 492251512 | 492251812 | 492252212 | 492252612 |
| 2.5 | 492500812 | 492501012 | 492501212 | 492501512 | 492501812 | 492502212 | 492502612 |
| 2.75 | 492750812 | 492751012 | 492751212 | 492751512 | 492751812 | 492752212 | 492752612 |
| 3.0 | 493000812 | 493001012 | 493001212 | 493001512 | 493001812 | 493002212 | 493002612 |
| 3.25 | 493250812 | 493251012 | 493251212 | 493251512 | 493251812 | 493252212 | 493252612 |
| 3.5 | 493500812 | 493501012 | 493501212 | 493501512 | 493501812 | 493502212 | 493502612 |
| 3.75 | 493750812 | 493751012 | 493751212 | 493751512 | 493751812 | 493752212 | 493752612 |
| 4.0 | 494000812 | 494001012 | 494001212 | 494001512 | 494001812 | 494002212 | 494002612 |
| 4.5 | 494500812 | 494501012 | 494501212 | 494501512 | 494501812 | 494502212 | 494502612 |
| 5.0 | 495000812 | 495001012 | 495001212 | 495001512 | 495001812 | 495002212 | 495002612 |
Compliance Chart
| Pressure (atm) | Balloon Diameter (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.0 | 2.25 | 2.5 | 2.75 | 3.0 | 3.25 | 3.5 | 3.75 | 4.0 | 4.5 | 5.0 | |
| 6 | 1.93 | 2.17 | 2.42 | 2.66 | 2.90 | 3.13 | 3.38 | 3.60 | 3.86 | 4.31 | 4.80 |
| 8 | 1.95 | 2.19 | 2.44 | 2.68 | 2.93 | 3.16 | 3.41 | 3.64 | 3.90 | 4.36 | 4.85 |
| 10 | 1.96 | 2.21 | 2.46 | 2.70 | 2.95 | 3.19 | 3.44 | 3.68 | 3.93 | 4.41 | 4.90 |
| 12 | 1.98 | 2.23 | 2.48 | 2.73 | 2.98 | 3.22 | 3.47 | 3.71 | 3.97 | 4.45 | 4.95 |
| 14 NP* | 2.00 | 2.25 | 2.50 | 2.75 | 3.00 | 3.25 | 3.50 | 3.75 | 4.00 | 4.50 | 5.00 |
| 16 | 2.02 | 2.27 | 2.52 | 2.77 | 3.02 | 3.28 | 3.53 | 3.79 | 4.03 | 4.55 | 5.05 |
| 18 | 2.04 | 2.29 | 2.54 | 2.80 | 3.05 | 3.31 | 3.56 | 3.82 | 4.07 | 4.59 | 5.10 |
| 20 RBP** | 2.05 | 2.31 | 2.56 | 2.82 | 3.07 | 3.34 | 3.59 | 3.86 | 4.10 | 4.64 | 5.15 |
| 22 RBP** | 2.07 | 2.33 | 2.58 | 2.84 | 3.10 | 3.37 | 3.62 | 3.90 | 4.14 | 4.69 | 5.20 |
| 24 RBP** | 2.09 | 2.35 | 2.60 | 2.86 | 3.12 | 3.40 | 3.65 | 3.93 | 4.17 | 4.73 | 5.25 |
| 26 | 2.11 | 2.37 | 2.62 | 2.89 | 3.15 | 3.43 | 3.67 | 3.97 | 4.21 | - | - |
| 28 | 2.12 | 2.39 | 2.64 | 2.91 | 3.17 | 3.46 | 3.70 | - | - | - | - |
*Nominal Pressure. The nominal in-vitro device specifications do not take into account any lesion resistance.
**Rated Burst Pressure. Do not exceed RBP.
Data on file at Abbott.
Sapphire and OrbusNeich are registered trademarks of OrbusNeich Medical Group Holdings Limited or its affiliates. Manufactured by OrbusNeich Medical Group Holdings Limited or its affiliates. Distributed by Cardiovascular Systems, Inc. (CSI). CSI is a subsidiary of the Abbott Group of Companies.
CAUTION: This OrbusNeich product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use at eifu.orbusneich.com for more detailed information on Indications, Contraindications, Warnings, Precautions, and Adverse Events.
MAT-2506878 v1.0
Important Safety Information
Sapphire‡ NC24 Coronary
Dilatation Catheter

INDICATIONS
The Sapphire‡ NC24 Coronary Dilatation Catheter is indicated for:
- Balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis in patients evidencing coronary ischemia for the purpose of improving myocardial perfusion.
- Balloon dilatation of a coronary artery occlusion for the treatment of acute myocardial infarction.
- In-stent restenosis.
- Post-delivery expansion of balloon expandable coronary stents.
CONTRAINDICATIONS
The use of Sapphire‡ NC24 Coronary Dilatation Catheter is contraindicated in the following patient types:
- Patients with an unprotected left main coronary artery.
- Patients with coronary artery spasm in the absence of a significant stenosis.
WARNINGS
When using this type of device, the following warnings should be observed:
- To reduce the potential for vessel damage, the inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis.
- PTCA in patients who are not acceptable candidates for coronary artery bypass graft surgery require careful consideration, including possible hemodynamic support during PTCA, as treatment of this patient population carries special risk.
- When the catheter is exposed to the vascular system, it should be manipulated while under high quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding.
- Balloon pressure should not exceed the Rated Burst Pressure (RBP) indicated on the package. The RBP is based on the results of in vitro testing. At least 99.9 percent of the balloons, (with a 95 percent confidence) will not burst at or below their rated burst pressure. Use of a pressure monitoring device is recommended to prevent over pressurization.
- PTCA should only be performed at hospitals where emergency coronary artery bypass graft surgery can be quickly performed in the event of a potentially injurious or life-threatening complication.
- Use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon.
- This device is designed and intended for single use only. DO NOT reprocess, re-sterilize and/or reuse. Reuse of single-use devices creates a potential risk of patient or user infections. Reuse may lead to impairment of functional performance. Infections and/or limited performance of the device may lead to injury, illness or death in the patient.
- Do not re-straighten a kinked hypotube; straightening a kinked metal shaft may result in breakage of the shaft.
PRECAUTIONS
- Use the catheter prior to the "Use By" date specified on the package.
- Prior to angioplasty, the dilatation catheter should be examined to verify functionality and ensure that its size is suitable for the specific procedure for which it is being used.
- The catheter system should be used only by physicians trained in percutaneous transluminal coronary angioplasty.
- During the procedure, appropriate anticoagulant and coronary vasodilator therapy must be provided to the patient as needed. After the procedure, anticoagulant therapy should be continued for a period of time as determined by the physician.
- Do not re-insert the PTCA catheter into the coil dispenser after procedural use.
- The design and construction of these catheters do not provide the user with distal pressure monitoring capability.
- Discard all disposable devices used during this procedure per local requirements for medical device waste disposal.
- Do not use oil-based contrast medium, organic solvents or alcohols; there is a possibility of catheter leak, damage or lubrication loss.
- Use with caution for procedures involving calcified lesions due to the abrasive nature of these lesions.
POTENTIAL COMPLICATIONS AND ADVERSE EVENTS
Potential complications and adverse effects due to the use of this product include, but are not limited to, the following:
- Death
- Acute myocardial infarction
- Acute Vessel Closure
- Total occlusion of the coronary artery or bypass graft
- Coronary vessel dissection, perforation, rupture, or injury
- Restenosis of the dilated vessel
- Hemorrhage or hematoma
- Unstable angina
- Arrhythmias, including ventricular fibrillation
- Drug reactions, allergic reaction to contrast medium
- Hypo/hypertension
- Infection
- Coronary artery spasm
- Arteriovenous fistula
- Stroke, air embolism and embolization of fragmentation of thrombotic or atherosclerotic material
CAUTION: This product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available), at manuals.eifu.abbott or at eifu.orbusneich.com for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events. This material is intended for use with healthcare professionals only.
MAT-2400959 v2.0

Stay Connected