Imaging and Diagnostic Systems
SensiTherm Multi Temperature Monitoring System
The SensiTherm™ Multi Temperature Monitoring System provides critical information necessary to adapt therapy where needed, enabling the physician to monitor temperatures in the esophagus between 0° C and 75° C.

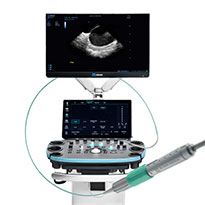
ViewMate Multi Ultrasound System & ViewFlex Xtra ICE Catheter
ViewMate™ Multi Ultrasound System with ViewFlex™ Xtra ICE Catheter provides clear visualization of intracardiac anatomy and accurate interpretation of blood flow direction and velocity.
MAT-2208661 v3.0
Indications, Safety, & Warnings
SensiTherm™ Multi Esophageal
Monitoring System
CAUTION: This product is intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available) or at manuals.sjm.com or eifu.abbottvascular.com for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events.
Indications for Use
The SensiTherm Multi Esophageal Monitoring System is composed of SensiTherm Multi Monitor and SensiTherm Multi Probe and is intended for the continuous detection, measurement and visualization (in °C) of esophageal temperature. The intended environments of use are operating rooms and interventional electrophysiology rooms. The SensiTherm Multi Probe must be used in conjunction with the SensiTherm Multi Monitor. The role of esophageal temperature monitoring using this device in reducing the risk of cardiac ablation related esophageal injury has not been established. The performance of the SensiTherm Multi system in detecting esophageal temperature changes as a result of energy delivery during cardiac ablation procedures has not been evaluated.
Contraindications
The insertion of the SensiTherm Multi Probe in the esophagus is not recommended if the esophagus is deformed or irritated. There are no specific contraindications or side effects related to the use of SensiTherm Multi Monitor. However, the insertion of the probe by an unskilled operator can cause discomfort and slight abrasions to the patient. To avoid that risk, it is essential that SensiTherm Multi Monitor is used only by medical personnel.
Warnings and Precautions
Thermal stabilization: The technology used by SensiTherm Multi Monitor for the metering process requires that the handle of the probe and that of the patient cable are approximately at the same temperature. Hence, it is recommended to wait at least 15 minutes between the instant in which the components are brought in the working environment and the start of a measurement session, especially if the probe or the patient cable have long stationed in environments with temperature and/or humidity significantly different from those of use. It is recommended not to place the two handles in such a way that during the measurements only one of the two objects remains for a prolonged time in proximity to or in contact with a heat or cold source. Failure to observe these precautions can lead to an accuracy of the instrument lower than the one stated in the technical specifications. MR Unsafe: Due to the presence of metallic parts in the components of SensiTherm® Multi Monitor, the device and its accessories must never be kept inside or in close proximity to a patient who is about to undergo magnetic resonance imaging. For the full list of warnings and precautions, please refer to the applicable Instructions for Use.
MAT-2208672 v1.0
ViewMate™ Multi Ultrasound System
Indications: ViewMate™ Multi Ultrasound System is applicable for adults, pregnant women, pediatric patients and neonates. It is intended for use in Abdominal, Intra-operative (abdominal, thoracic, and vascular), Pediatric, Small Organ (Thyroid, Breast, Testes), Neonatal Cephalic, Adult Cephalic, Musculo-skeletal (Conventional, Superficial), Cardiac Adult, Cardiac Pediatric, Trans-esoph. (Cardiac), Intra-cardiac, and Peripheral vessel exams. Modes of operation include: B, M, PWD, CWD, Color Doppler, Amplitude Doppler, Combined mode (B+M, PW+B, Color+B, Power+B, PW+Color+B, Power+PW+B), Tissue Harmonic Imaging, TDI, Color M. This device is a general purpose diagnostic ultrasound system intended for use by qualified and trained healthcare professionals for ultrasound imaging, measurement, display and analysis of the human body and fluid, which is intended to be used in a hospital or medical clinic.
Contraindications: The diagnostic ultrasound system is not intended for ophthalmic use.
Warnings: Do not use an aftermarket probe other than those specified by Mindray. The probes may damage the system causing a profound failure, e.g. a fire in the worst case. Reference manual for full list of warnings..
Precautions: This system must be used only by qualified medical professionals. Reference manual for full list of precautions.
MAT-2302213 v2.0
ViewFlex™ X ICE Catheter
Indications of Use
The ViewFlex™ X ICE Catheter Sensor Enabled™ is indicated for use in adult and adolescent pediatric patients for intra-cardiac and intra-luminal visualization of cardiac and great vessels anatomy and physiology, as well as visualization of other devices in the heart. When used with a compatible three-dimensional mapping system, the catheter provides location information. The ViewFlex™ X ICE Catheter Sensor Enabled™ is contraindicated if there is an occurrence of conditions which create unacceptable risk during catheterization. if the patient has a mechanical tricuspid valve (a prosthetic tissue valve is permissible). in patients with an active systemic infection as this may increase the risk for cardiac infection. when there is a presence of deep venous thrombosis or abnormalities or when there is no adequate vascular access. for use in the left side of the heart for patients unable to receive heparin, or an acceptable alternative, to achieve adequate anticoagulation. when there is a presence of a known intracardiac thrombus in a chamber, other chambers are open to use, and via the transseptal approach in patients with left atrial thrombus or myxoma, or interatrial baffle or patch. in the coronary vasculature due to risk of damage to the coronary arteries. The ViewFlex™ X ICE Catheter Sensor Enabled™ is to be used only with the ViewFlex™ Catheter Interface Module and the ViewMate™ Multi ultrasound consoles. Any other use or inappropriate electrical connection may pose a serious risk to patient safety. The ViewFlex™ X ICE Catheter Sensor Enabled™ includes a 9 F shaft. The physician should consider anatomical size restrictions if considering use of the ViewFlex™ X ICE Catheter Sensor Enabled™. Do not use excessive force to advance or withdraw the catheter. Using excessive force can result in patient injury or death. Ensure that the two steering knobs are in the neutral position before advancing or withdrawing the catheter. If you encounter strong resistance during catheter articulation, discontinue the procedure. Identify and address the cause of the resistance before resuming the procedure. Withdraw and redirect the catheter as needed. Have antiarrhythmic drugs, an external defibrillator, and respiratory assist equipment available in case of complications during the use of this device. Catheter materials are not compatible with magnetic resonance imaging (MRI). Arrhythmia, bleeding, cardiac Perforation, cardiovascular injury, cerebrovascular injury, electric shock, embolism, immunological reaction, infection, myocardial ischemia, organ injury, peripheral vascular injury, respiratory compromise.
