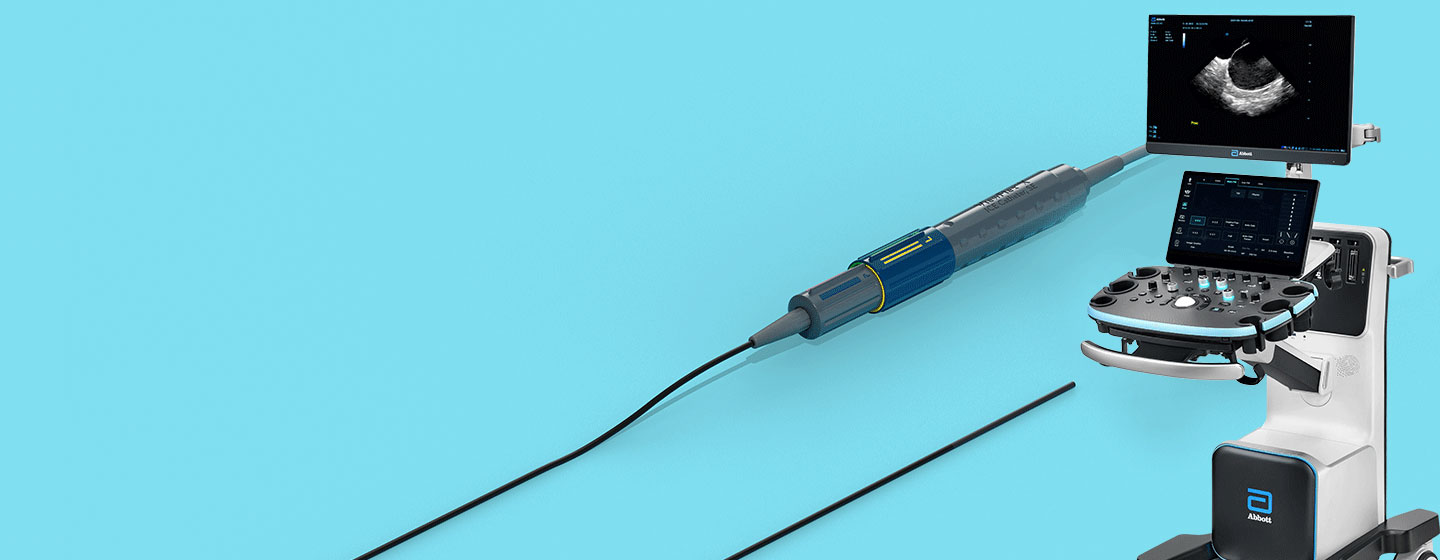
Use the ViewMate™ Multi Ultrasound System & ViewFlex™ X ICE Catheter, Sensor Enabled™ to visualize cardiac structures, blood flow and devices within the heart.
Increased Productivity with a Fully Featured System
The ViewMate Multi Ultrasound System delivers superb image quality across the entire field and intuitive touch-screen capabilities, all on a robust platform built with capabilities.
- Features comprehensive imaging capabilities that can eliminate the need for additional imaging equipment being transported to the lab1
- Multi-transducer port connects four transducers simultaneously, which allows for quick selection of transducer based on clinical need (ICE, Transthoracic, Transesophageal and Vascular)
- Choose multiple imaging modes (2-D, M-Mode, Pulsed Wave, Continuous Wave, Color Doppler, Tissue Doppler Imaging*)
- Designed to support future lab integration and ICE technology advancements**
Streamline Procedures Single-Handedly1
The ViewFlex™ X ICE Catheter, SE provides seamless maneuverability, designed to allow physicians to focus more on the procedure and less on catheter manipulation to enhance workflow efficiency.

Reduce Complications with ICE-Guided Ablation
Cardiac Complications2
Such as pericardial effucsion, tamponade, thrombus formation, pulmonary vein stenosis, with microbubbles detected as a result of overheating.3

Cardiac Perforations (0.25% vs 1.3%)2

In-Hospital Mortality (0.72 hazard ratio)4

*When paired with a ViewFlex™ X ICE Catheter, SE
**With compatible software version
References
- Abbott. Data on File. Report 90094426.
- Aldhoon B, et al. Complications of catheter ablation for atrial fibrillation in a high-volume centre with the use of intracardiac echocardiography. Europace (2013) 15, 24–32.
- Goya M, et al. The use of intracardiac echocardiography cathers in endocardial ablation of cardiac arrhythmia: Meta-analysis of efficiency, effectiveness, and safety outcomes. J Cardiovasc Electrophysiol. 2020;31:664-673.
- Isath A, et al. Does the use of intracardiac echocardiography during atrial fibrillation catheter ablation improve outcomes and cost? A nationwide 14-year analysis from 2001 to 2014. Journal of Interventional Cardiac Electrophysiology 61 (3) p.461-468.
Rx Only. Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events, and directions for use.
United States: Required Safety Information
MAT-2302212 v3.0
Indications, Safety & Warnings
ViewMate™ Multi Ultrasound System
Indications: ViewMate™ Multi Ultrasound System is applicable for adults, pregnant women, pediatric patients and neonates. It is intended for use in Abdominal, Intra-operative (abdominal, thoracic, and vascular), Pediatric, Small Organ (Thyroid, Breast, Testes), Neonatal Cephalic, Adult Cephalic, Musculo-skeletal (Conventional, Superficial), Cardiac Adult, Cardiac Pediatric, Trans-esoph. (Cardiac), Intra-cardiac, and Peripheral vessel exams. Modes of operation include: B, M, PWD, CWD, Color Doppler, Amplitude Doppler, Combined mode (B+M, PW+B, Color+B, Power+B, PW+Color+B, Power+PW+B), Tissue Harmonic Imaging, TDI, Color M. This device is a general purpose diagnostic ultrasound system intended for use by qualified and trained healthcare professionals for ultrasound imaging, measurement, display and analysis of the human body and fluid, which is intended to be used in a hospital or medical clinic.
Contraindications: The diagnostic ultrasound system is not intended for ophthalmic use.
Warnings: Do not use an aftermarket probe other than those specified by Mindray. The probes may damage the system causing a profound failure, e.g. a fire in the worst case. Reference manual for full list of warnings..
Precautions: This system must be used only by qualified medical professionals. Reference manual for full list of precautions.
MAT-2302213 v2.0
ViewFlex™ X ICE Catheter
Indications of Use
The ViewFlex™ X ICE Catheter Sensor Enabled™ is indicated for use in adult and adolescent pediatric patients for intra-cardiac and intra-luminal visualization of cardiac and great vessels anatomy and physiology, as well as visualization of other devices in the heart. When used with a compatible three-dimensional mapping system, the catheter provides location information. The ViewFlex™ X ICE Catheter Sensor Enabled™ is contraindicated if there is an occurrence of conditions which create unacceptable risk during catheterization. if the patient has a mechanical tricuspid valve (a prosthetic tissue valve is permissible). in patients with an active systemic infection as this may increase the risk for cardiac infection. when there is a presence of deep venous thrombosis or abnormalities or when there is no adequate vascular access. for use in the left side of the heart for patients unable to receive heparin, or an acceptable alternative, to achieve adequate anticoagulation. when there is a presence of a known intracardiac thrombus in a chamber, other chambers are open to use, and via the transseptal approach in patients with left atrial thrombus or myxoma, or interatrial baffle or patch. in the coronary vasculature due to risk of damage to the coronary arteries. The ViewFlex™ X ICE Catheter Sensor Enabled™ is to be used only with the ViewFlex™ Catheter Interface Module and the ViewMate™ Multi ultrasound consoles. Any other use or inappropriate electrical connection may pose a serious risk to patient safety. The ViewFlex™ X ICE Catheter Sensor Enabled™ includes a 9 F shaft. The physician should consider anatomical size restrictions if considering use of the ViewFlex™ X ICE Catheter Sensor Enabled™. Do not use excessive force to advance or withdraw the catheter. Using excessive force can result in patient injury or death. Ensure that the two steering knobs are in the neutral position before advancing or withdrawing the catheter. If you encounter strong resistance during catheter articulation, discontinue the procedure. Identify and address the cause of the resistance before resuming the procedure. Withdraw and redirect the catheter as needed. Have antiarrhythmic drugs, an external defibrillator, and respiratory assist equipment available in case of complications during the use of this device. Catheter materials are not compatible with magnetic resonance imaging (MRI). Arrhythmia, bleeding, cardiac Perforation, cardiovascular injury, cerebrovascular injury, electric shock, embolism, immunological reaction, infection, myocardial ischemia, organ injury, peripheral vascular injury, respiratory compromise.