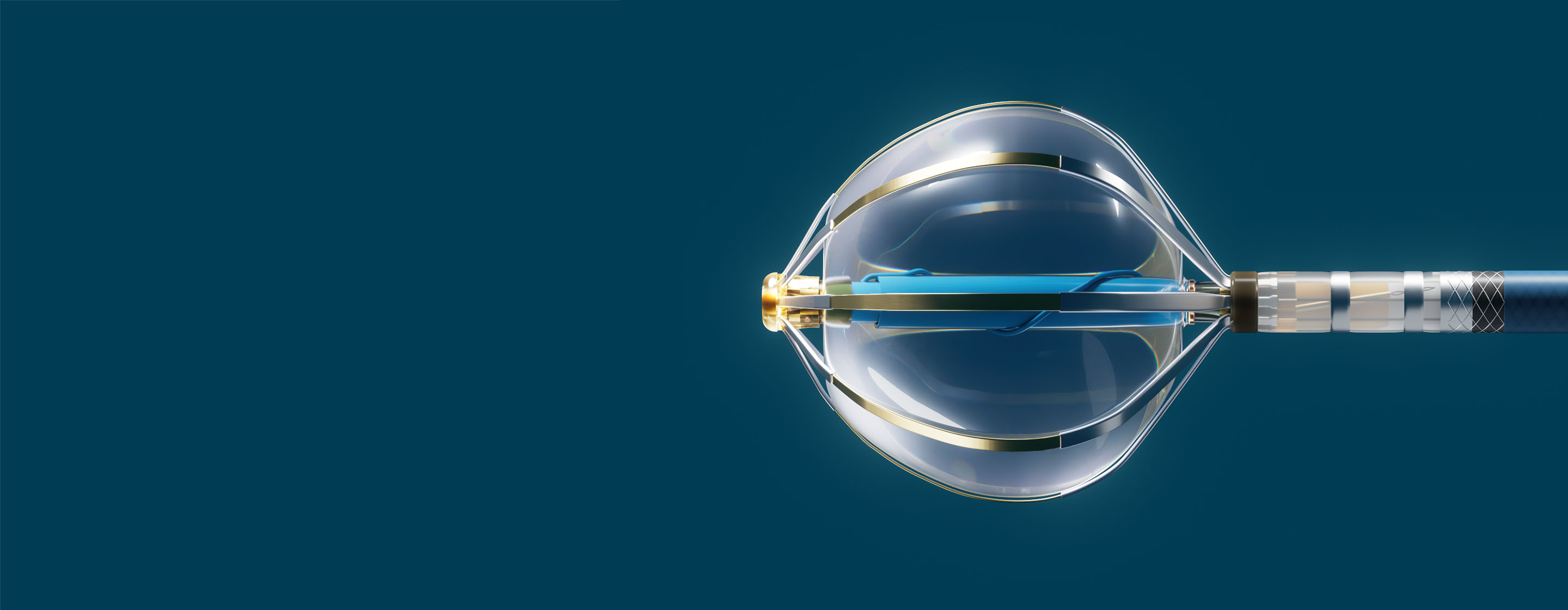
Volt™ PFA System
The Volt™ PFA System consists of the following components:
- Volt™ PFA Catheter, Sensor Enabled™: A novel balloon-filled basket with 8 active splines. It is designed for therapy delivery, pacing, and the collection of electrical and anatomical data when used with the EnSite™ X EP System.
- Current™ PFA Generator: The streamlined user interface includes waveform selection, tissue proximity LivePoint™ display, electrode selectivity, and therapy count tracking. Abbott's Current PFA Generator is designed for intuitive use and is built to be extensible for future PFA catheter types.
- Agilis™ NxT Steerable Introducer (13 F): The best-in-class Agilis™ platform now in a 13 F inner diameter for use with larger French size catheters. In addition, the Volt PFA System is compatible with a 13 F inner diameter introducer such as Agilis NxT Steerable Introducer. Visit the product page.
Latest data released at AF Symposium 2026
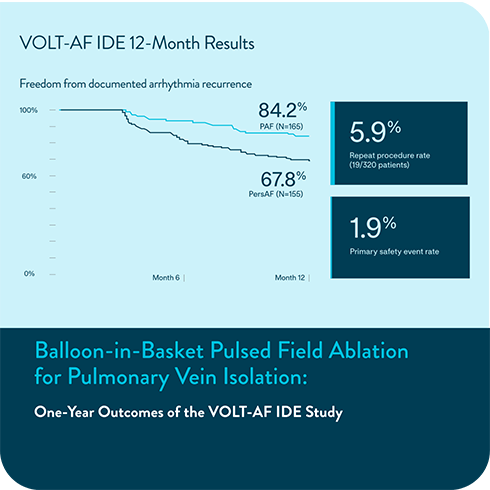
New data from the VOLT-AF IDE Study describe the use of a balloon‑in‑basket pulsed field ablation system for patients with PAF and PersAF. The study reports on 12 month outcomes, procedural characteristics, patient outcomes, and measures of quality of life. The platform has been designed as an option for de novo PVI.
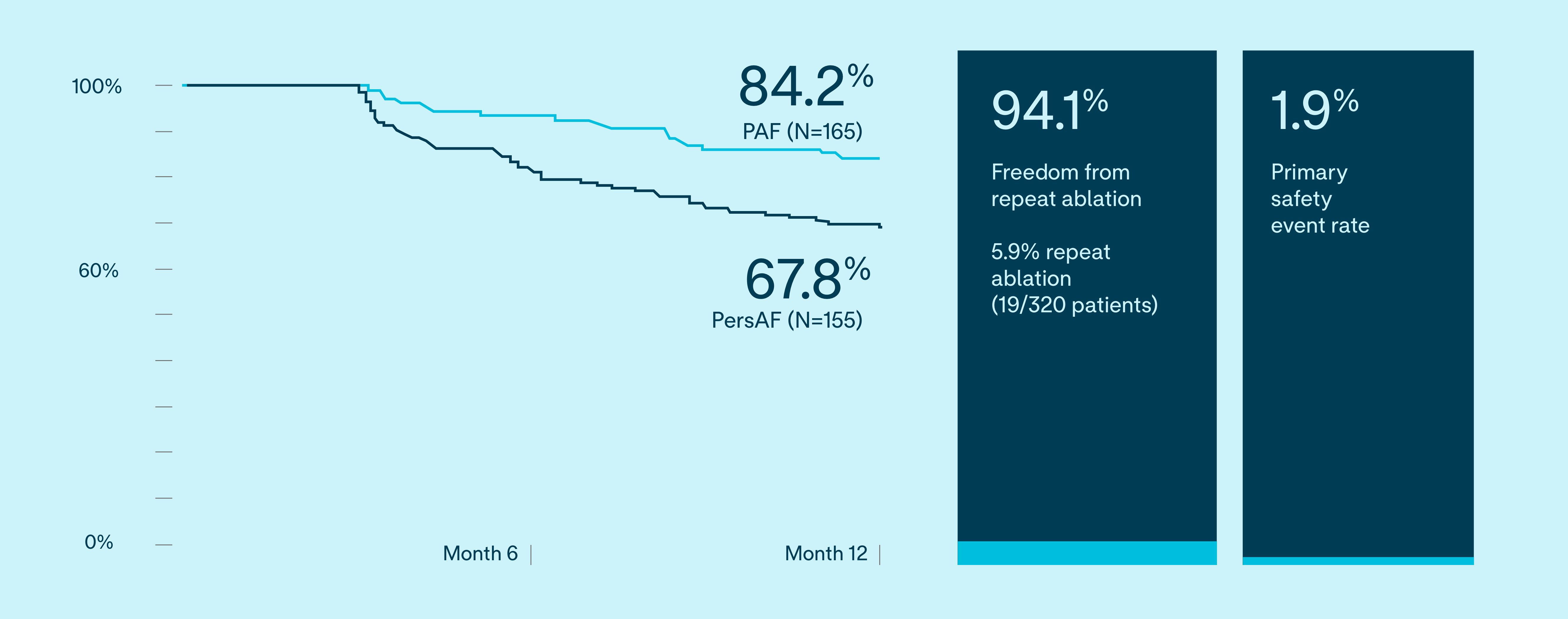
12-month IDE results evaluate safety and effectiveness1
VOLT-AF IDE 12-Month Results
Freedom from documented AF/AT/AFL recurrence
VOLT IDE 12 month results
Freedom from documented AF/AT/AFL
Efficacy was not impacted by:
(p>0.05)
- Conscious/deep sedation use
- Fluoroscopy use
- Catheter learning curve/physician experience
- Low Waveform use
Subjects free from composite, protocol-defined primary effectiveness endpoint failure
81.1%
PAF (N=165)
63.3%
PersAF (N=155)
The primary effectiveness endpoint was freedom from composite acute procedural failure and documented atrial fibrillation (AF), atrial flutter (AFL), or atrial tachycardia (AT) episodes lasting longer than 30 seconds, occurring after the 90-day blanking period and through 12 months of follow-up.
These results reinforce the Volt PFA System’s ability to deliver safe, effective, and lasting outcomes—helping clinicians treat confidently while supporting long term patient wellbeing.
Data Released at APHRS/JHRS 2025
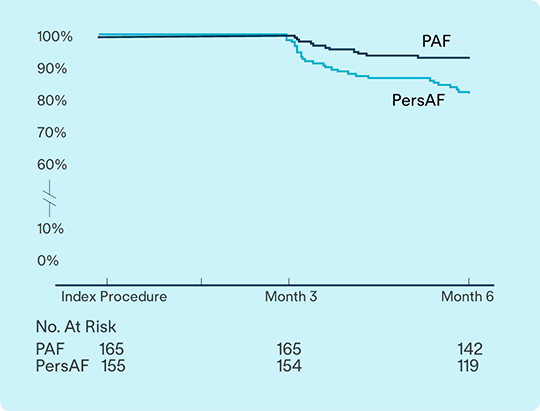
6-Month IDE results confirm strong safety and effectiveness2
Data from VOLT-AF IDE study evaluates safety and effectiveness for treatment of Paroxysmal AF (PAF) and Persistent AF (PsAF).2
93.1%
of PAF
81.9%
of PsAF
subjects free from documented arrhythmia recurrence at 6 months
1.9%
Primary safety endpoint
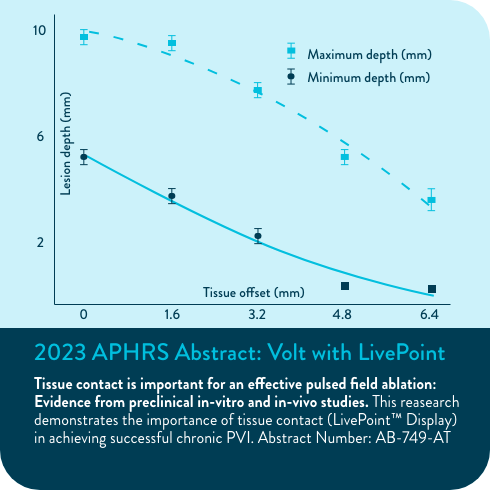
Zero-fluoroscopy workflows3
Procedures performed with zero fluoroscopy during the VOLT-AF IDE study demonstrated comparable safety and effectiveness to traditonal workflows*3
93.8%
first-pass isolation was achieved (N=15/16)
100%
acute effectiveness in the zero-fluoro group
0%
safety events
(*N=16/320)
Real-world use: single catheter, deep sedation4
First real-world experience with Volt PFA System was presented on its procedural efficiency and adaptability4, with outcomes consistent with clinical trial data:
73.5%
of cases used Volt PFA System for both mapping and ablation
70.6%
of cases were completed without the use of general anesthesia (GA)

Posterior wall ablation*5
First reported cases using Volt PFA System for posterior wall ablation show5:
100%
acute success 87/87
0%
safety events
Volt PFA System Clinical Studies
Volt CE Mark Study
Before being used with patients, the Volt PFA System underwent extensive laboratory testing. Following this, the Volt CE Mark Study was the first clinical research study to investigate the Volt PFA System's safety and performance in treating human patients. The purpose of this study was to gather data demonstrating that the Volt PFA System functions as intended in a clinical setting and to establish its safety and effectiveness for treating atrial fibrillation.

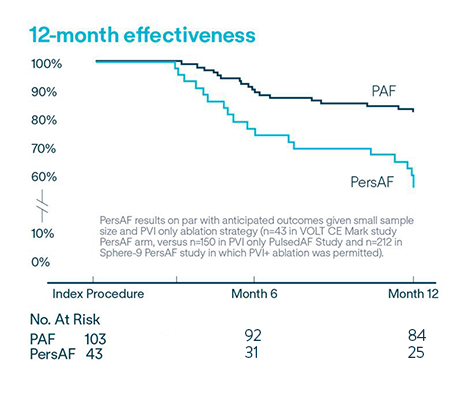
CE Mark 12-Month Results6
VOLT CE Mark Study: Long-term safety and effectiveness of de novo PVI in treating AF.
Protocol required PVI only left atrial ablation with the Volt™ PFA System
Effectiveness
of PAF (n=103) subjects free from documented AF/AFL/AT recurrence at 12-months6
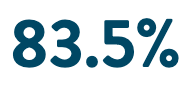
Repeat ablation and durably isolated PVs6
94.5% freedom from repeat ablation (5.5% rate of repeat ablation; n=8/146)
90.0% of veins were durably isolated in repeat ablations after the blanking period
Primary safety endpoint
- 0% of subjects reporting hemolysis, acute kidney injury, phrenic nerve injury6
- 2.7% of subjects experienced a primary safety endpoint event6
58.1% of PersAF (n=43) subjects free from documented AF/AFL/AT recurrence at 12-months6
VOLT-AF IDE and VOLT CE Mark Studies
Subjects under conscious sedation (CS) and deep sedation (DS) workflows experienced acute effectiveness with no significant difference in safety when compared to general anesthesia (GA) procedures.7,8
CS/DS workflows, compared to GA showed
↓18%
less time in the lab (31.9 min)7
↓14%
lower procedure time (16 min)7
↓33%
lesstime in the LA (15.8 min)7
Acute effectiveness
Safety
98.5%
GA subjects (255/259)7
100%
CS/DS subjects (56/56) (P=1.000)7
0%
primary safety events observed in subjects without propofol7,8
VOLT-AF IDE and VOLT CE Mark Analyses
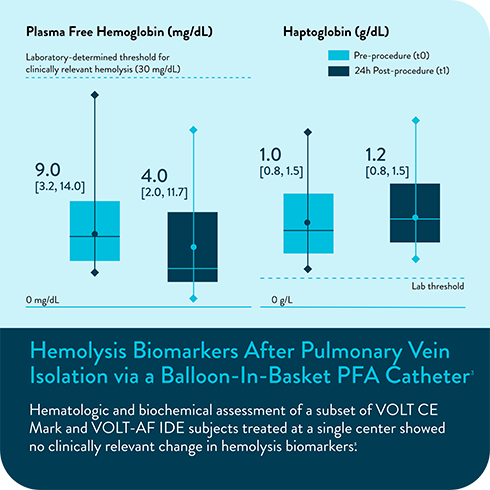
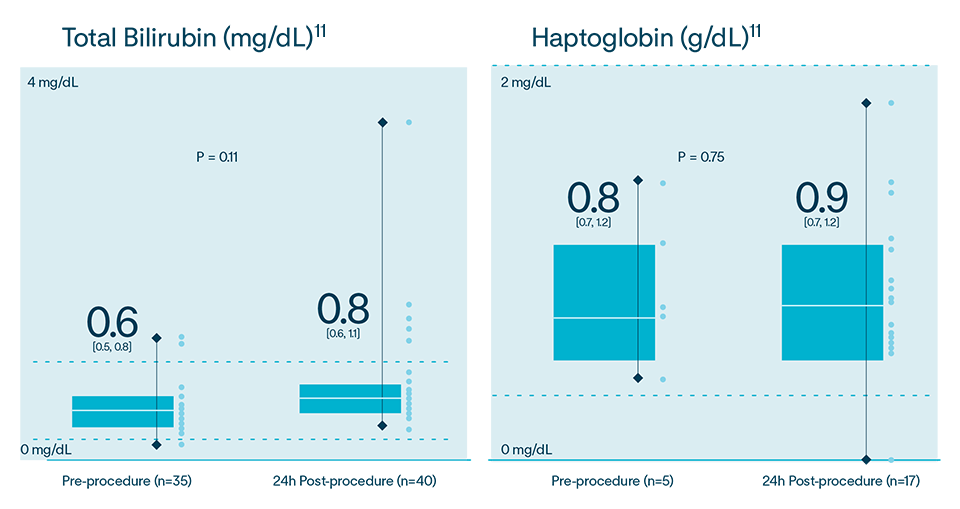
In multiple analyses, no clinically relevant hemolysis was observed, and biomarkers did not exhibit statistically significant change9-11

Hemolysis or kidney injury reported in either VOLT-AF IDE or VOLT CE Mark studies10,11
VOLT-AF IDE Analysis
CE Mark Feasibility Sub-Study Results
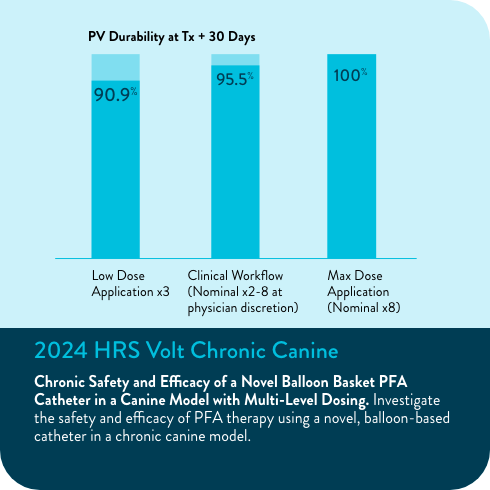
In the Volt CE Mark feasibility sub-study, acute effectiveness was achieved in 99.2% (127/128) of treated PVs (96.9% of subjects, 31/32) with 23.8 ± 4.2 PFA applications/subject. No esophegeal lesions causally related to Volt™ PFA System.
Volt AF IDE Study
This clinical research study is intended to demonstrate safety and effectiveness of the Volt™ PFA System for the treatment of symptomatic, recurrent, drug-refractory paroxysmal and persistent atrial fibrillation.
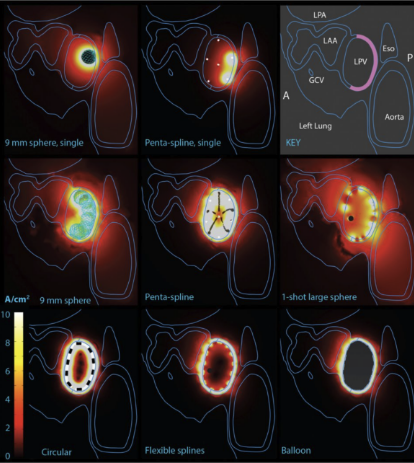
Computer Modeling of PFA Waveform Design
2024 Manuscript Assessing PFA Design Considerations
Comparison of efficiency of PFA catheter designs by computer modeling. Computer models demonstrate a wide range in efficiency among PFA catheters. Form factors such as exposure of PFA electrodes to blood pool significantly influence efficiency. Higher efficiency designs, such as balloon-based designs, have less collateral current.
- Verma, A., et al. (2026, February 5) 12-Month Safety and Effectiveness of a balloon-in-basket PFA system for de novo PVI to treat PAF and PersAF: Results from the VOLT-AF IDE Study [Late Breaking Presentation]. AF Symposium 2026, Boston MA, USA
- Late Breaker presentation at APHRS 2025. Safety and Effectiveness of a Novel Balloon-Based PFA System to Treat PAF and PersAF: 6-Month Results of the VOLT-AF IDE Study (Sanders, et al).
- Sundaram, S. et al. (2025, November 15). Outcomes of Zero-Fluoroscopy Procedures with the Volt PFA Catheter [Short session]. Presented by Prash Sanders. Asia Pacific Heart Rhythm Society (APHRS) 2025, Yokohama, Japan.
- Puererfellner, H. et al. (2025, November). First Real-World Report of Experience with a Novel Balloon-in-Basket Pulsed Field Ablation Catheter [Oral presentation]. Presented by Helmut Puererfellner. Asia Pacific Heart Rhythm Society (APHRS) 2025, Yokohama, Japan.
- Chierchia, G. et al. (2025, November). First Reported Cases of Posterior Wall Ablation Using a Balloon-in-Basket PFA Catheter [Oral presentation]. Presented by Helmut Puererfellner. Asia Pacific Heart Rhythm Society (APHRS) 2025, Yokohama, Japan.
- Tilz, R., et al. (2025, April 26). Long-Term Safety and Effectiveness of balloon-based PFA system for de novo PVI to treat PAF and PersAF: Results from the VOLT CE Mark Study [Oral presentation]. Presented by Prash Sanders. Heart Rhythm Society 2025, San Diego, CA.
- Acute safety and procedural characteristics of conscious and deep sedation to general anesthesia workflows with novel balloon-based PFA system (Oral presentation and abstract by Roland Tilz, EHRA 2025).
- Lo, M., et al. (2025, April 26). Procedural efficiency, safety and acute effectiveness of conscious and deep sedation workflows with novel balloon-based PFA system [Poster presentation]. Heart Rhythm Society 2025, San Diego, CA.
- Marcon L, Della Rocca DG, Vetta G, et al. Hemolysis Biomarkers After Pulmonary Vein Isolation via a Balloon-In-Basket PFA Catheter. Circulation. Online Version of Record before inclusion in an issue. doi:10.1161/CIRCULATIONAHA.124.070333.
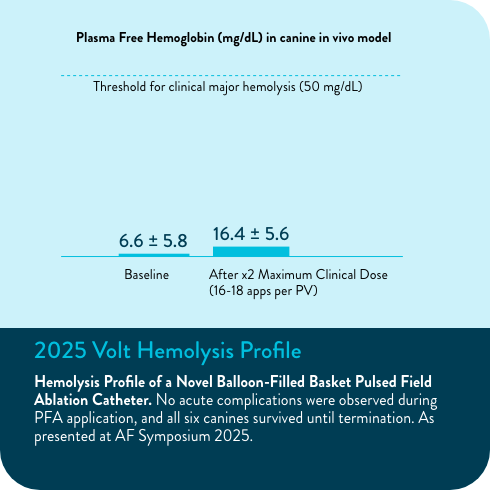
- Overmann JA, Marques M, Lafean C, Pipenhagen C, Moon BL, Verma A. Hemolysis Profile of a Novel Balloon-Filled Basket Pulsed Field Ablation Catheter. Poster presented at: AF Symposium 2025; 2025 Jan 15-17; Boston, MA.
- Woods, C., et al. (2025, April 26). Impact of pulsed field ablation using a balloon-in-basket catheter on hemolysis and renal function biomarkers [Poster presentation]. Heart Rhythm Society 2025, San Diego, CA.
- Tilz, R.R. (2025, January 17) Acute results demonstrate safety and effectiveness of balloon-based pulsed field ablation system for de novo PVI in PAF and PersAF [Late Breaking Presentation]. AF Symposium 2025, Boston MA, USA.
- Hemolysis Biomarkers After Pulmonary Vein Isolation via a Balloon-In- Basket PFA Catheter. https://doi.org/10.1111/jce.16608.
MAT-2500133 v10.0
Rx Only. Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events, and directions for use.
United States: Required Safety Information
Indications for Use: The Volt™ PFA Catheter, Sensor Enabled™ is indicated for the treatment of symptomatic, recurrent, drug-refractory paroxysmal or persistent (episode duration less than one year) atrial fibrillation when used in conjunction with a compatible pulsed field ablation (PFA) generator. The catheter is compatible with the EnSite™ X EP System. Contraindications: The Volt™ PFA Catheter, Sensor Enabled™ is contraindicated for: ▪ Patients who have had a ventriculotomy or atriotomy within the preceding four weeks. ▪ Patients with prosthetic valves as the catheter may damage the prosthesis. ▪ Patients with an active systemic infection as this may increase the risk for cardiac infection. ▪ Patients with a myxoma or an intracardiac thrombus as the catheter could precipitate an embolus. ▪ Patients unable to receive heparin or an acceptable alternative to achieve adequate anti-coagulation. The transseptal approach is contraindicated in a patient with an interatrial baffle or patch because the opening could persist and produce an iatrogenic atrial shunt. The retrograde trans-aortic approach is contraindicated in patients who have had aortic valve replacement. Warnings: Misuse of this device may result in serious complications. Do not alter this device in any way. This device is intended for one time use only; do not reprocess or reuse it. Note the product “Use by” date on the package. Any attempt to resterilize and reuse this system may compromise its integrity. Adverse effects of using nonsterile components may include, but are not limited to: ▪ Local or systemic infection or reaction ▪ Mechanical damage ▪ Inaccurate functionality. For both patients and laboratory staff, cardiac catheterization procedures present the potential for significant x-ray exposure, which can result in acute radiation injury as well as increased risk for somatic and genetic effects due to the x-ray beam intensity and duration of the fluoroscopic imaging. Carefully consider the use of this device in pregnant women. This device should be used by physicians trained in the techniques of catheter ablation in a fully equipped electrophysiology laboratory. Do not immerse the proximal handle or cable connectors in fluids; electrical performance could be affected. Do not use excessive force to advance or withdraw the catheter when resistance is encountered. Vascular perforation or dissection is an inherent risk of any catheter placement. Careful catheter manipulation must be performed to avoid device component damage, thromboembolism, cerebrovascular accident, cardiac damage, perforation, pericardial effusion, or tamponade. The safety and long-term effects of lesions created by pulse field ablation have not been established. In particular, the long-term effects of lesions in proximity to the specialized conduction system or coronary vasculature are unknown. The safety and effectiveness of the device has not been established in pregnant women or pre-pubescent children. Careful consideration must therefore be given for the use of the device in pregnant women or prepubescent children. Concurrent use of non-linear catheters within the atrial chamber may increase the risk of entanglement. Catheter entanglement with other device is a possible complication of electrophysiology procedures. To unentangle the Volt™ PFA Catheter, Sensor Enabled™, deflate the balloon and manipulate the opposing catheter to the center of the basket electrodes. Follow any relevant entanglement procedures from the opposing device. Aspiration during catheter introduction or withdrawal is recommended to limit the potential for air ingress into the patient. During catheter preparation, rinsing or submerging the basket in saline reduces the potential for blood coagulation. During catheter preparation, shifting the straightener over the basket removes any residual air remaining in the folds of the balloon and reduces the potential for air embolism. Use of a syringe larger than 12 mL for balloon inflation increases the risk of overinflation. This can result in detachment of the spline electrodes at the distal coupler. Pacemakers and implantable cardioverter/defibrillators can be affected adversely by PFA signals. It is important to: ▪ Have temporary external sources of pacing and defibrillation available during ablation. ▪ Deactivate ICDs because they can discharge and injure the patient or even damage the ICDs during the ablation procedure. ▪ Exercise extreme caution while ablating near atrial or ventricular permanent pacing leads. ▪ Perform complete pacing system analysis on all patients after ablation. Precautions: Do not deflect the introducer while the basket is within the deflectable portion of the introducer, as this can cause damage to the introducer deflection mechanism. Do not deflect the Volt™ PFA Catheter, Sensor Enabled™ while the basket is in the introducer, as this can cause damage to the catheter deflection mechanism. The first application of PFA will likely result in a significant reduction of amplitude of PV potentials recorded from the electrodes on the catheter. Do not use this as an immediate indication that no further ablation is necessary. The PFA therapy should be delivered in accordance with the Therapy Delivery Parameters. Advance the catheter slowly through the introducer to minimize the risk of air embolism. Do not attempt to use the device before completely reading and understanding the applicable instructions for use. Inspect the package prior to use. Do not use if the packaging or catheter appears damaged. Inspect all components before use. Excessive bending or kinking of the catheter may cause damage to the catheter. Be careful not to twist the electrodes with respect to the catheter shaft; twisting may damage the electrode bond and loosen the electrodes. Release the steering (make the catheter straight) prior to pulling back the catheter into the introducer. Deflate the balloon before pulling the catheter back into the introducer. Overinflation of the balloon may cause damage to the device. Always straighten the catheter shaft and keep the balloon deflated before insertion or withdrawal. Do not use if the catheter appears damaged, kinked, or if there is difficulty in deflecting the distal section to achieve the desired curve. Do not use if the catheter does not hold its curve or if there is difficulty deflecting the catheter. Do not use if there is difficulty inflating or deflating the balloon. Do not use excessive force to advance or withdraw the catheter while inside the introducer when resistance is encountered to avoid potential damage to the catheter. Do not expose catheter to organic solvents such as alcohol. Catheter advancement must be performed with support from visualization modalities such as Intracardiac echo, fluoroscopy, and or compatible navigation and visualization systems to minimize the risk of cardiac damage, perforation, or tamponade. If other catheters are used concomitantly with the Volt™ PFA Catheter, Sensor Enabled™, only use other catheters in close proximity when the balloon is inflated, and never in tandem with the catheter in the same pulmonary vein. Never retract the catheter into the introducer when another catheter is in close proximity. Never use a loop catheter concomitantly with the Volt™ PFA Catheter, Sensor Enabled™ within the same atrial chamber. Retract the catheter into the introducer before using a loop catheter within the same chamber. Prior to therapy delivery, ensure that the guidewire is not touching the basket electrodes to prevent ineffective therapy. Compliance with intended therapy delivery parameters reduces the potential for hemolysis and acute kidney injury. Ensure that vacuum pressure is relieved and balloon is fully deflated. After deflating the balloon, leave the stopcock open between the syringe and the inflation lumen to prevent catheter damage during catheter withdrawal. It is recommended to withdraw the guidewire into the introducer prior to balloon deflation to avoid potential entanglement with spline electrodes. Ensure even spacing for splines and full balloon inflation to reduce potential for generator fault detection. Ensure connection to center lumen stopcock when attempting to inject contrast. The balloon inflation lumen has a blue/grey striped sleeve to visually distinguish it from the center lumen stopcock. Do not touch the guidewire during therapy delivery. Risk of electric shock to the user is possible under rare circumstances in which the guidewire is in contact with an active therapy electrode(s) and the user. During treatment with the catheter, ensure there is no possibility of contact with electrodes from another catheter. To avoid thromboemboli, intravenous heparin should be used when entering the left heart during ablation. Consult the HRS consensus guidelines for anticoagulation strategies pre-, during, and post-catheter ablation. Individual patient anatomy and physician technique may require procedural variations. Maintain an activated clotting time (ACT) of greater than 300 seconds at all times during use of the catheter. Store in a dry place. After use, the device accessories and packaging should be appropriately classified for disposal, e.g. biohazard, sharps, non-hazardous waste etc., and carefully disposed of in compliance with facility procedures and applicable laws and regulations. Catheter materials are not compatible with magnetic resonance imaging (MRI). Potential Adverse Events: The potential adverse events may be related to the ablation catheter(s) and/or the interventional procedure. The severity and/or the frequency of these potential adverse events may vary and may result in prolonged procedure time and/or additional medical and/or surgical intervention, implantation of a permanent device such as a pacemaker, and in rare cases, may result in death. The following adverse events have been documented for catheter ablation procedures: ▪ Abnormal vision ▪ Acute kidney injury ▪ Air embolism ▪ Anesthesia reaction ▪ Angina/chest pain/discomfort ▪ Aorto-right atrial fistula ▪ Arrhythmias, including exacerbation of preexisting atrial fibrillation ▪ Arteriovenous fistula ▪ AV/SA node stunning (asystole) ▪ Bleeding, including major bleeding requiring surgery or transfusion/hematomas/anemia ▪ Cardiac perforation/tamponade ▪ Cardiac embolism ▪ Cardiovascular injury, including atrial trauma and coronary artery/pulmonary vein trauma ▪ COPD exacerbation ▪ Component damage to Implantable Cardioverter Defibrillator(ICD) or implantable pacemaker ▪ Coronary artery spasm ▪ Cytotoxicity/systemic toxicity/sensitization/endotoxin/pyrogen ▪ Death ▪ Dislodgement of ICD or pacing leads ▪ Electrical shock ▪ Endocarditis ▪ Esophageal lesion ▪ Fever ▪ Foreign body embolism ▪ Heart block/unintended ablation ▪ Heart failure ▪ Hemothorax ▪ Hypotension ▪ Infectious pericarditis ▪ Left atrial esophageal fistula ▪ Myocardial infarction ▪ Neck/back/groin/chest pain/discomfort (general chest pain not associated with MI) ▪ Palpitations ▪ Pericarditis ▪ Pericardial effusion ▪ Phrenic nerve injury ▪ Peripheral vascular dissection/laceration ▪ Pleural effusion ▪ Pneumonia ▪ Pneumothorax ▪ Pseudoaneurysm ▪ Pulmonary edema ▪ Pulmonary embolism ▪ Pulmonary hypertension ▪ Pulmonary vein stenosis ▪ Radiation injury ▪ Respiratory failure/distress/depression/hypoxia ▪ Silent cerebral event/lesion ▪ Stiff Left Atrial Syndrome ▪ Stroke/cerebrovascular accident ▪ Syncope/vasovagal reaction/dizziness ▪ Transient ischemic attack (TIA) ▪ Thromboembolism ▪ Thrombosis/thrombus ▪ Vagal nerve injury including Gastroparesis ▪ Valvular damage or insufficiency
Indications: The EnSite™ X EP System is a suggested diagnostic tool in patients for whom electrophysiology studies have been indicated. The EnSite™ X EP System provides information about the electrical activity of the heart and displays catheter location during conventional electrophysiological (EP) procedures. Warnings: For patient safety, any connections that directly connect the patient to the EnSite™ X EP System must be routed through the appropriate modules: EnSite™ X EP System SurfaceLink Module, EnSite™ X EP System 20 pin Catheter Input Module, EnSite™ X EP System 80-pin Catheter Input Module and Direct Connect Ports on the EnSite™ X EP System Amplifier. When using the EnSite™ X EP System, full protection against the effects of cardiac defibrillator discharge and other leakage currents is dependent upon the use of appropriate cables. Refer to the ablation catheter IFU for a listing of adverse events related to the use of this device in conjunction with ablation, as a part of the diagnosis and treatment of cardiac arrhythmias. Non-SE catheters cannot collect location data and should not be used for navigation in VoXel Mode because they do not have a magnetic sensor. However, they can be visualized and display intracardiac signals. Only connect items that have been specified as part of the EnSite X EP System or compatible with the EnSite X EP System to the multiple socket-outlets. The EnSite™ X EP System model display should be used in conjunction with conventional EP techniques to confirm catheter location. The AutoMark feature does not indicate lesion effectiveness. AutoMarks are placed based on user-defined parameters for catheter stability and RF metrics only. PFA AutoMarks are placed based on electrode location and user-defined PFA metrics only. Sudden impedance changes of the body or catheter electrodes caused by the connection of other devices (e.g., stimulator, defibrillator, and other devices) may create a location shift. The 2D and 3D LivePoint Displays should not be used as the primary / sole display of tissue proximity during an Electrophysiology study. Refer to the Current PFA Generator IFU for warnings related to the Volt ™ LivePoint Display. Precautions: Ensure that surface electrodes, Patient Reference Sensors, and associated connectors do not contact one another, electrical ground, or metallic objects. Do not operate the EnSite™ X EP System Field Frame within 10 m of another operating Field Frame. Do not place the EnSite™ X EP System Field Frame Cable inside the measurement volume or wrap it around the EnSite™ X EP System Field Frame, as it may create a magnetic interference. Metallic equipment used in close proximity to the magnetic field during the procedure, such as a sterile drape holder, may cause metal distortion. Do not place tool cables within 30 mm of the EnSite™ X EP System Field Frame Cable. If placed this close-particularly if the cables are parallel to each other the tool cable may become subject to electromagnetic interference. Do not use the EnSite™ X EP System for magnetic catheter localization or magnetic data collection (NavX SE or EnSite VoXel point collection) if other magnetic fields are present.
Indications: The Agilis™ NxT Steerable Introducer Dual-Reach™ is indicated for the introduction of various cardiovascular catheters into the heart, including the left side of the heart, during the treatment of cardiac arrhythmias. Contraindications: The Agilis™ NxT Steerable Introducer Dual-Reach™ is contraindicated for: Previous interatrial septal patch. Known or suspected atrial myxoma. Acute myocardial infarction. Unstable angina. Recent cerebral vascular accident (CVA). Patients who do not tolerate anticoagulation therapy. Patients with an active infection. Presence of an intracardiac thrombus. Warnings: Do not alter this device in any way. This device must be used by board-certified electrophysiologists, or EP fellows in training, in a fully-equipped operational EP laboratory. This device is intended for one time use only; do not reprocess or reuse it. Note the product “Use by” date on the package. Any attempt to resterilize and reuse this system may compromise its integrity. Adverse effects of using nonsterile components may include, but are not limited to: Local or systemic infection or reaction, Mechanical damage, Inaccurate functionality. Always aspirate, insert and withdraw components, and exchange catheters slowly to minimize the risk of air emboli. Aspirate all air before fluid infusion from the sideport. Provide continuous heparinized saline infusion while the introducer remains in the vessel. Fibrin may accumulate in or on the introducer tip during the procedure. To prevent dislodgement of potential thrombus, aspirate when removing dilator or catheter. Before removing the steerable introducer, reinsert the guidewire through the introducer, reintroduce the dilator over the guidewire, straighten the steerable introducer, then remove the dilator, guidewire, and introducer as a unit. Maximum in-vivo time: 7 hours. Read the IFU carefully before using this device to help reduce the potential risks and complications associated with the transseptal technique, such as air emboli and perforation of the aorta and left atrium. Aspirate and saline flush the introducer frequently to minimize the potential for thrombus formation. Do not use the introducer without a catheter or dilator supporting the lumen. Use of the introducer directly over a wire without a catheter or dilator supporting the lumen may result in complications that can cause death. For both patients and laboratory staff, cardiac catheterization procedures present the potential for significant x-ray exposure, which can result in acute radiation injury as well as increased risk for somatic and genetic effects due to the x-ray beam intensity and duration of the fluoroscopic imaging. Carefully consider the use of this introducer in pregnant women. Persons with known history of allergies to any of the materials listed below may suffer an allergic reaction to this device. Before use, counsel the patient on the materials contained in the device and discuss a thorough history of allergies. This device contains: Polyether block amide (PEBAX), Polytetrafluoroethylene, ABS, Silicone rubber, DOW Corning 360 fluid , HDPE, MDX/hexane solution, Nylon. Precautions: Federal law (U.S.) restricts this device to sale by or on the order of a physician. Only use this device with equipment that complies with international safety standards. Store in a cool, dark, dry place. Inspect all components before use. Do not use if the packaging or items in the kit appear to be damaged or defective. Conditions requiring special consideration when using this product may be, but are not limited to, small left atrium, marked right atrial enlargement, and marked distortion of the thorax configuration (example, kyphosis or scoliosis). Individual patient anatomy and physician technique may require procedural variations. Potential Adverse Events: The following potential complications may occur during the use of this device, but are not limited to: Arrhythmia. Bleeding Major: bleeding requiring surgery or transfusion, Hematomas or Anemia. Cardiac perforation: Cardiac tamponade, Pericardial complications, Pericardial effusion Hemopericardium, Pneumopericardium, Pericarditis. Cardiovascular injury: Atrial/ventricular trauma, Great vessel perforation, Valvular damage. Cerebral injury: Asymptomatic cerebral emboli (ACE), Stroke/cerebrovascular accident, Transient ischemic attack (TIA). Coronary artery injury. Embolism: Air embolism, Foreign body embolism, Pulmonary embolism, Thromboembolism, Thrombosis/thrombus. Hypotension: Vasovagal reaction. Immunological reaction: Anesthesia reaction, Anaphylaxis. Infection: Endocarditis, Pneumonia, Sepsis/shock. Organ injury: Esophageal injury, Pleural effusion. Pain: Groin. Peripheral vascular injury: Arteriovenous fistula, Dissection, Laceration, Pseudoaneurysm. Superficial tissue injury. Please consult the respective manufacturer's labeling for adverse events associated with the use of either cardiovascular catheters or endomyocardial biopsy devices.
Indications for Use: The Current™ PFA Generator is indicated for use with compatible ablation catheters for the treatment of cardiac arrhythmias. Contraindications: There are no known contraindications specific to the use of the Current™ PFA Generator itself. However, users should read and understand the specific indications, contraindications, warnings, and precautions included with any cardiac ablation catheter used in conjunction with the Current™ PFA Generator. Warnings: Misuse of this device may result in serious complications. This device must be used by, or under the supervision of, physicians trained in the techniques of percutaneous electrophysiology studies. For both patients and laboratory staff, cardiac catheterization procedures present the potential for significant Xray exposure, which can result in acute radiation injury as well as increased risk for somatic and genetic effects due to the Xray beam intensity and duration of the fluoroscopic imaging. Carefully consider the use of this device in pregnant women. The safety and effectiveness of the device is not established in pregnant women or prepubescent children. Carefully consider the use of this device in pregnant women or prepubescent children. Pacemakers and implantable cardioverter/defibrillators can be affected adversely by PFA signals. It is important to: Have temporary external sources of pacing and defibrillation available during ablation. Deactivate ICDs because they can discharge and injure the patient or even damage the ICDs during the ablation procedure. Exercise extreme caution while ablating near atrial or ventricular permanent pacing leads. Perform complete pacing system analysis on all patients after ablation. Ablation within and near the coronary arterial vasculature is associated with myocardial infarction and death. The long-term risk of lesions created by PFA is not established. When using an EP recording system, the equipment must be front-end isolated or have an isolated patient cable and comply with the IEC 606011 safety requirements for medical electrical systems. Failure of the Current™ PFA Generator can result in an unintended power output increase or the inability to remove power when commanded. In case of system malfunction, attempt to stop PFA delivery by pressing the Standby button on the front panel or turning off the power switch on the rear panel. If those attempts do not turn off the power, disconnect the power cord. Failure to do so can result in operator or patient harm. The Current™ PFA Generator is not suitable for use within Oxygen Rich Environments or in the presence of flammable gasses, including flammable anesthetic mixtures with air, oxygen, or nitrous oxide. Avoid using this equipment adjacent to or stacked with other equipment because it can result in improper operation. If such use is necessary, observe all equipment involved to verify that they are operating normally. To avoid the risk of electric shock, only connect this equipment to a mains power supply with protective earth. Use of accessories, transducers, and cables other than those specified can result in improper operation due to increased electromagnetic emissions or decreased electromagnetic immunity of this equipment. Do not use portable RF communications equipment (including peripherals, such as antenna cables and external antennas) closer than 30 cm (12 in.) to any part of the Current™ PFA Generator (including cables specified by the manufacturer) because it can result in degradation of the equipment. During treatment, ensure there is no possibility of contact with electrodes from another catheter. Do not open the Current™ PFA Generator in attempt to access its internal components. Opening the device may result in personal injury and damage to the generator. Do not position the Current™ PFA Generator so that it is difficult to disconnect the equipment from the mains supply. Do not modify the Current™ PFA Generator. Precautions: Federal (USA) law restricts this device to sale by or on order of a physician. Do not attempt to operate the Current™ PFA Generator before reading this IFU thoroughly. Read, understand, and follow the Current™ PFA Generator IFU carefully. For future reference, keep this IFU in a convenient, readily accessible place. During use of the catheter in the left side of the heart, therapeutic anticoagulation needs to be achieved. Anticoagulation treatment must adhere to the ESC/AHA/ACC or any other consensus guidelines to avoid thromboemboli. Do not immerse cable connectors in fluids; electrical performance can be affected adversely. The Current™ PFA Generator defaults to the nominal waveform unless the user selects the lower waveform. The Current™ PFA Generator is capable of delivering high voltage. Improper handling of the catheter can result in patient or operator injury, particularly when operating the device. During high voltage delivery, do not allow the patient to contact grounded metal surfaces. Place a non-conductive material between the patient and grounded metal surfaces. Electrode attachments are to be as close to the operating field as possible. Place connecting cables so contact with the patient or other leads is avoided. Advance the catheter with support from visualization modalities such as intracardiac echo (ICE), fluoroscopy, and compatible navigation and visualization systems to minimize the risk of cardiac damage, perforation, or tamponade. Accessory equipment connected to the analog and digital interfaces must comply with the respective IEC standards (IEC 60950 for data-processing equipment and IEC 606011 for medical equipment). Apparent low voltage output or failure of the equipment to function correctly at normal settings may indicate failure of an electrical lead. Do not increase the voltage before checking for obvious defects or misapplication. Place the unit so the airflow around it is adequate for cooling. Inspect and test reusable cables and accessories regularly. Only connect items that are specified as part of the system. To reduce the risk of accidental burns, avoid skin to skin contact (for example, between the arms and body of the patient) by inserting dry gauze. Place monitoring electrodes as far as possible from surgical electrodes when surgical equipment and physiological monitoring equipment are used simultaneously. This equipment generates, uses, and can radiate energy. If not installed and used in accordance with the instructions, this equipment may be interfered with by, or cause harmful interference to, other equipment in the vicinity, even if the other equipment complies with CISPR emission requirements. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to devices, which can be determined by turning the equipment off and on, the user is encouraged to correct the interference by one or more of the following measures:
Reorient or relocate the receiving device. Increase separation between the equipment. Connect the equipment into an outlet on a circuit different from that to which the other device(s) are connected. Consult the manufacturer for help. Follow the system installation to achieve optimal use. In the event of an error or warning message, check the programmed ablation parameters against the specific ablation catheter IFU before continuing delivery of ablation energy to the patient. To ensure electrical safety, do not use the USB ports to connect this device to other nonAbbott Medical powered devices. When using nonAbbott Medical devices, the USB ports should only be used with port powered devices such as a USB flash drive, keyboard, or mouse. The USB ports are not electrically isolated. Do not touch the USB port and the patient simultaneously. To ensure electrical safety, do not touch the HDMI ports and the patient simultaneously; the HDMI ports are not electrically isolated. External monitors/ switches/hubs that are nonAbbott Medical devices must be IEC 62368 compliant. If the system is used with an uninterruptible power supply, this device should not be connected to an external monitor. The user is responsible for compliance with IEC 606011 safety requirements for medical electrical systems and IEC 606011 Edition 3.1, Clause 16 when connecting this device to other devices. Monitoring systems incorporating high-frequency current-limiting devices are recommended. Studies have shown that smoke generated during electrocautery procedures can be potentially harmful to patients and the surgical team. These studies recommend adequately ventilating the smoke by using a surgical-smoke evacuator or other means. Do not operate or position the Current™ PFA Generator and its accessories within the vicinity of magnetic resonance imaging (MRI) equipment. The Current™ PFA Generator and its accessories have not been evaluated for safety, functionality, or compatibility within the MRI environment. Interaction with MRI equipment may adversely impact the Current™ PFA Generator's performance, degrade MRI quality, and result in patient harm. Do not clean the electrical contacts/pins of the connectors. Do not clean system components with bleach. Do not apply cleaners while the system is warm to the touch. Do not sterilize system components. Do not immerse system components in liquid. Turn off and disconnect power to the Current™ PFA Generator before cleaning it. Do not autoclave any Current™ PFA Generator component. Autoclaving may damage the system components. Dispose of/recycle packaging materials according to any applicable facility procedures and local regulations. At the end of useful life, classify the hardware for disposal/recycle (example: electrical equipment, non-hazardous waste, and so forth) and dispose of/recycle the hardware according to any applicable facility procedures and local regulations. The IFU is recyclable.
Adverse Events
| Adverse Event Category | Adverse Event Subcategory |
| Arrhythmia | Exacerbation of preexisting atrial fibrillation |
| Heart block/unintended ablation | |
| Palpitations | |
| AV/SA node stunning (asystole) | |
| Bleeding | Major bleeding requiring surgery or transfusion |
| Hematoma | |
| Anemia | |
| Cardiac perforation | Tamponade |
| Pericardial effusion | |
| Pericarditis | N/A |
| Cardiovascular injury | Atrial trauma and coronary artery/pulmonary vein trauma |
| Valvular damage or insufficiency | |
| Peripheral vascular injury | Dissection |
| Laceration | |
| Arteriovenous fistula | |
| Pseudoaneurysm | |
| Pain | Neck |
| Back | |
| Groin | |
| Chest (general pain not associated with MI) | |
| Cerebral injury | Stroke/cerebrovascular accident |
| Silent cerebral event/lesion | |
| Transient ischemic attack (TIA) | |
| Electrical shock | N/A |
| Embolism | Air embolism |
| Foreign body embolism | |
| Pulmonary embolism | |
| Thromboembolism | |
| Thrombosis/thrombus | |
| Cardiac embolism | |
| Heart failure decompensation | Heart failure |
| Pulmonary edema | |
| Hypotension | Syncope/vasovagal reaction/dizziness |
| Immunological reaction | Anesthesia reaction |
| Cytotoxicity/systemic toxicity/sensitization/endotoxin/pyrogen | |
| Infection | Endocarditis |
| Fever | |
| Infectious pericarditis | |
| Pneumonia | |
| Myocardial ischemia | Angina/chest pain/discomfort |
| Myocardial infarction | |
| Organ injury | Aorto-right atrial fistula |
| Left atrial esophageal fistula | |
| Esophageal lesion | |
| Pleural effusion | |
| Pneumothorax | |
| Hemothorax | |
| Acute kidney injury | |
| Peripheral nerve damage | Phrenic nerve injury |
| Respiratory compromise | Pulmonary hypertension |
| Respiratory failure/distress/depression/hypoxia | |
| Death | N/A |
| Abnormal vision | N/A |
| Coronary artery spasm | N/A |
| COPD exacerbation | N/A |
| Pulmonary vein stenosis | N/A |
| Radiation injury | N/A |
| Stiff left atrial syndrome | N/A |
| Vagal nerve injury including gastroparesis | N/A |
| Component damage to implantable cardioverter defibrillator (ICD) or implantable pacemaker | N/A |
| Dislodgement of ICD or pacing leads | N/A |