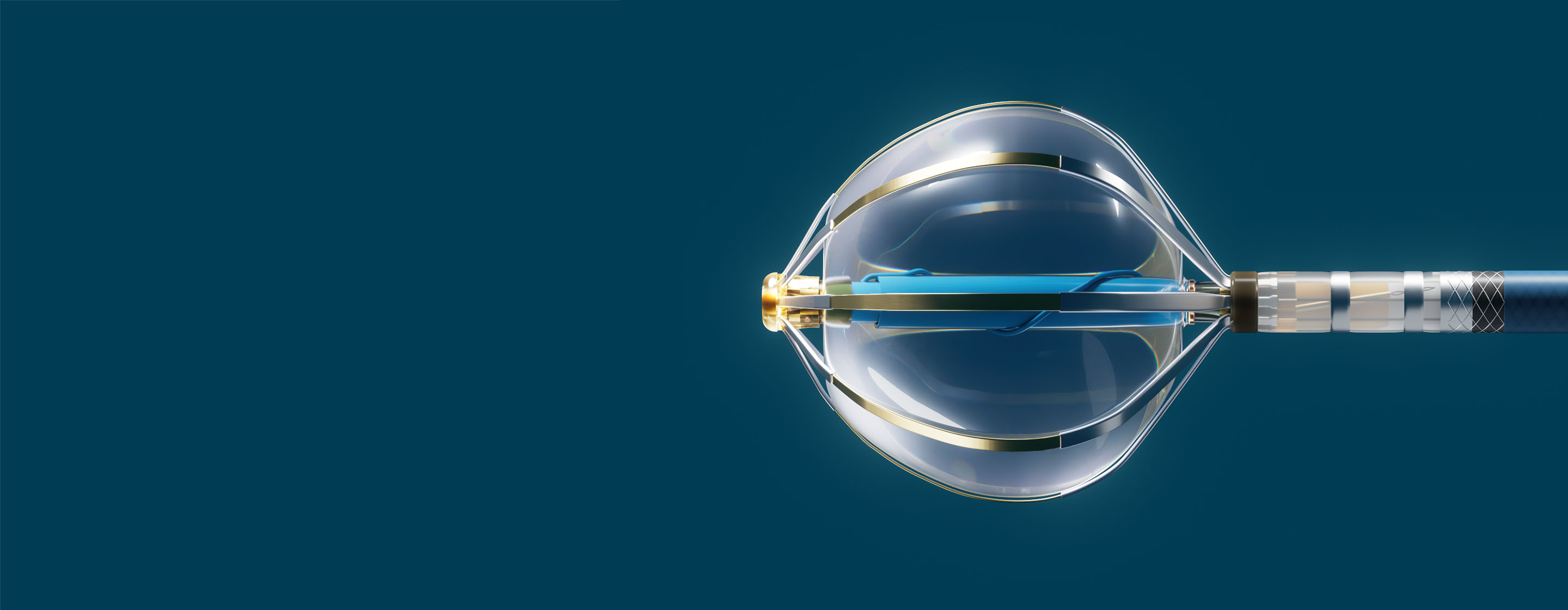
Volt™ PFA System
The Volt™ PFA System consists of the following components:
- Volt™ PFA Catheter, Sensor Enabled™: A novel balloon-filled basket with 8 active splines. It is designed for therapy delivery, pacing, and the collection of electrical and anatomical data when used with the EnSite™ X EP System.
- Current™ PFA Generator: The streamlined user interface includes waveform selection, tissue proximity LivePoint™ display, electrode selectivity, and therapy count tracking. Abbott's Current PFA Generator is designed for intuitive use and is built to be extensible for future PFA catheter types.
- Agilis™ NxT Steerable Introducer (13 F): The best-in-class Agilis™ platform now in a 13 F inner diameter for use with larger French size catheters. In addition, the Volt PFA System is compatible with a 13 F inner diameter introducer such as Agilis NxT Steerable Introducer. Visit the product page.
Latest data released at AF Symposium 2026
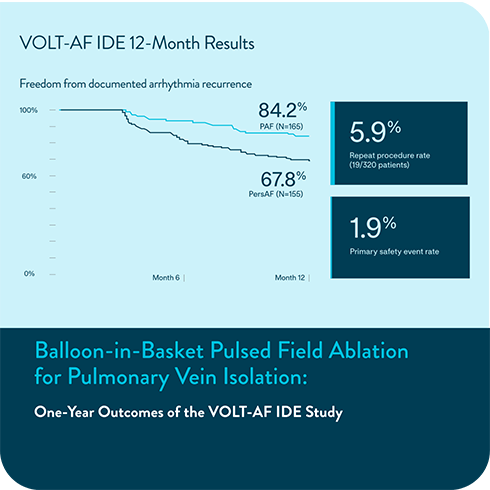
New data from the VOLT-AF IDE Study describe the use of a balloon‑in‑basket pulsed field ablation system for patients with PAF and PersAF. The study reports on 12 month outcomes, procedural characteristics, patient outcomes, and measures of quality of life. The platform has been designed as an option for de novo PVI.
12-month IDE results evaluate safety and effectiveness1
VOLT-AF IDE 12-Month Results
Freedom from documented AF/AT/AFL recurrence
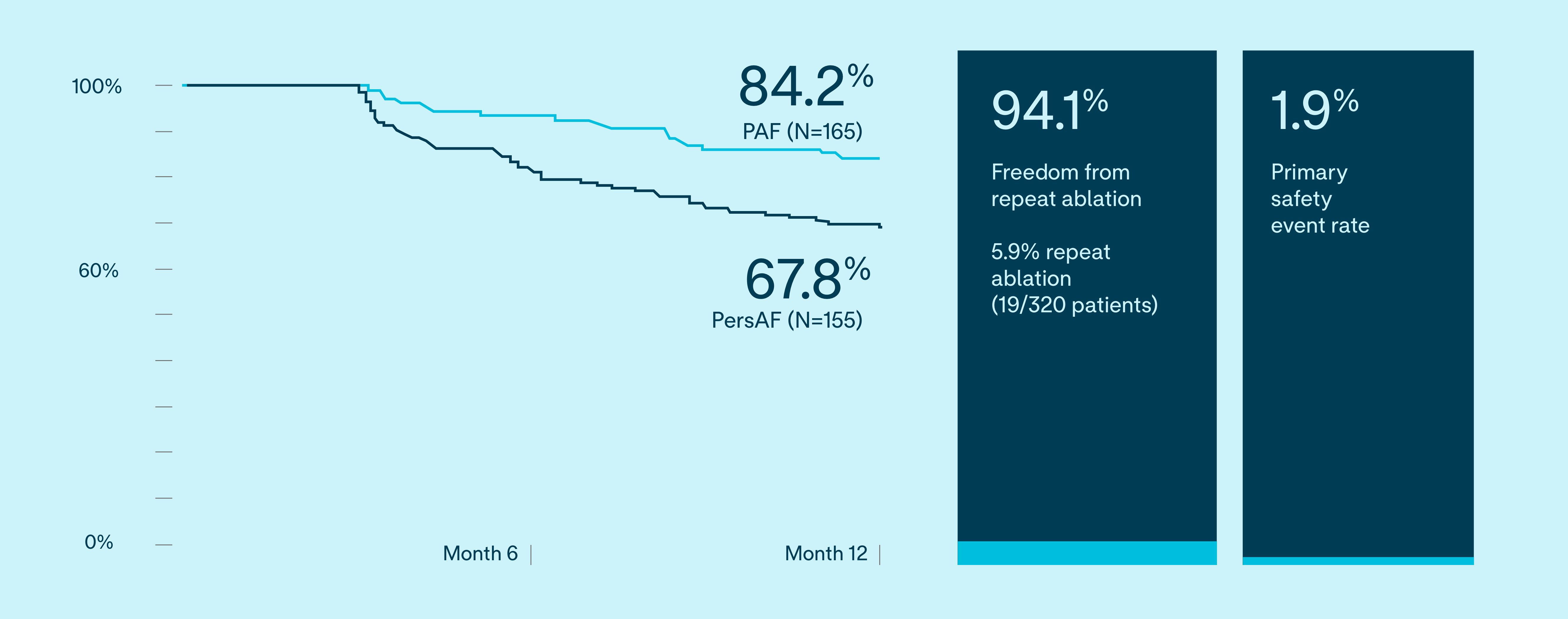
VOLT IDE 12 month results
Freedom from documented AF/AT/AFL
Efficacy was not impacted by:
(p>0.05)
- Conscious/deep sedation use
- Fluoroscopy use
- Catheter learning curve/physician experience
- Low Waveform use
Subjects free from composite, protocol-defined primary effectiveness endpoint failure
81.1%
PAF (N=165)
63.3%
PersAF (N=155)
The primary effectiveness endpoint was freedom from composite acute procedural failure and documented atrial fibrillation (AF), atrial flutter (AFL), or atrial tachycardia (AT) episodes lasting longer than 30 seconds, occurring after the 90-day blanking period and through 12 months of follow-up.
These results reinforce the Volt PFA System’s ability to deliver safe, effective, and lasting outcomes—helping clinicians treat confidently while supporting long term patient wellbeing.
Data Released at APHRS/JHRS 2025
6-Month IDE results confirm strong safety and effectiveness2
Data from VOLT-AF IDE study evaluates safety and effectiveness for treatment of Paroxysmal AF (PAF) and Persistent AF (PsAF).2
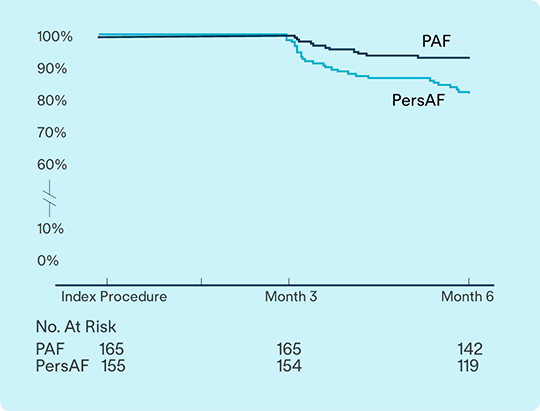
93.1%
of PAF
81.9%
of PsAF
subjects free from documented arrhythmia recurrence at 6 months
1.9%
Primary safety endpoint
Zero-fluoroscopy workflows3
Procedures performed with zero fluoroscopy during the VOLT-AF IDE study demonstrated comparable safety and effectiveness to traditonal workflows*3
93.8%
first-pass isolation was achieved (N=15/16)
100%
acute effectiveness in the zero-fluoro group
0%
safety events
(*N=16/320)
Real-world use: single catheter, deep sedation4
First real-world experience with Volt PFA System was presented on its procedural efficiency and adaptability4, with outcomes consistent with clinical trial data:
73.5%
of cases used Volt PFA System for both mapping and ablation
70.6%
of cases were completed without the use of general anesthesia (GA)

Posterior wall ablation*5
First reported cases using Volt PFA System for posterior wall ablation show5:
100%
acute success 87/87
0%
safety events
Volt PFA System Clinical Studies
Volt CE Mark Study
Before being used with patients, the Volt PFA System underwent extensive laboratory testing. Following this, the Volt CE Mark Study was the first clinical research study to investigate the Volt PFA System's safety and performance in treating human patients. The purpose of this study was to gather data demonstrating that the Volt PFA System functions as intended in a clinical setting and to establish its safety and effectiveness for treating atrial fibrillation.

CE Mark 12-Month Results6
VOLT CE Mark Study: Long-term safety and effectiveness of de novo PVI in treating AF.
Protocol required PVI only left atrial ablation with the Volt™ PFA System
Effectiveness
of PAF (n=103) subjects free from documented AF/AFL/AT recurrence at 12-months6
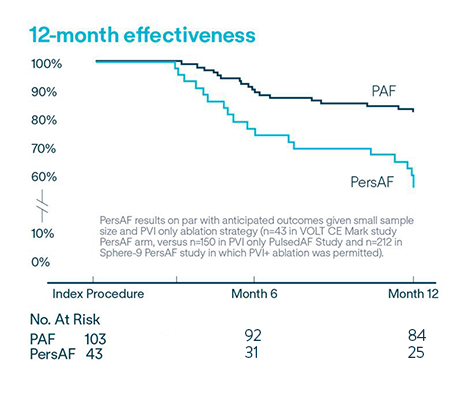
Repeat ablation and durably isolated PVs6
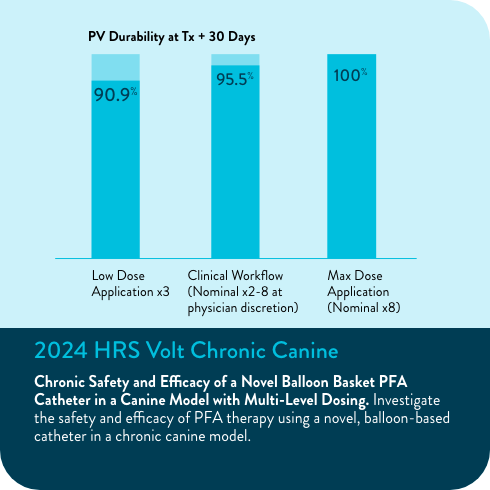
94.5% freedom from repeat ablation (5.5% rate of repeat ablation; n=8/146)
90.0% of veins were durably isolated in repeat ablations after the blanking period
Primary safety endpoint
- 0% of subjects reporting hemolysis, acute kidney injury, phrenic nerve injury6
- 2.7% of subjects experienced a primary safety endpoint event6
58.1% of PersAF (n=43) subjects free from documented AF/AFL/AT recurrence at 12-months6
VOLT-AF IDE and VOLT CE Mark Studies
Subjects under conscious sedation (CS) and deep sedation (DS) workflows experienced acute effectiveness with no significant difference in safety when compared to general anesthesia (GA) procedures.7,8
CS/DS workflows, compared to GA showed
↓18%
less time in the lab (31.9 min)7
↓14%
lower procedure time (16 min)7
↓33%
lesstime in the LA (15.8 min)7
Acute effectiveness
Safety
98.5%
GA subjects (255/259)7
100%
CS/DS subjects (56/56) (P=1.000)7
0%
primary safety events observed in subjects without propofol7,8
VOLT-AF IDE and VOLT CE Mark Analyses
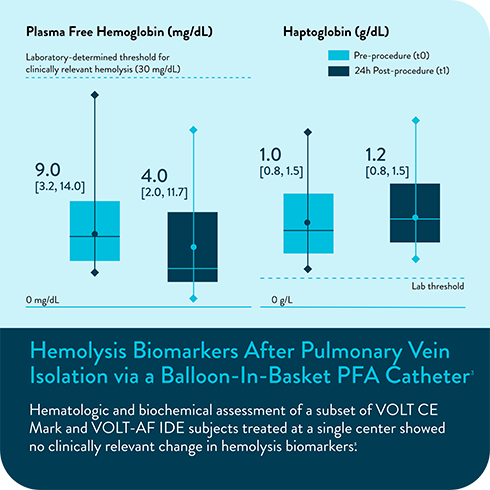
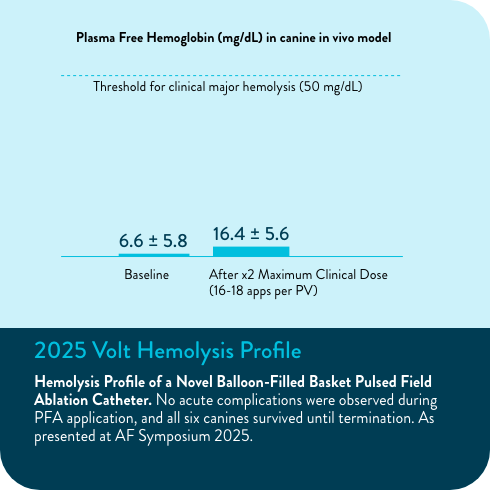
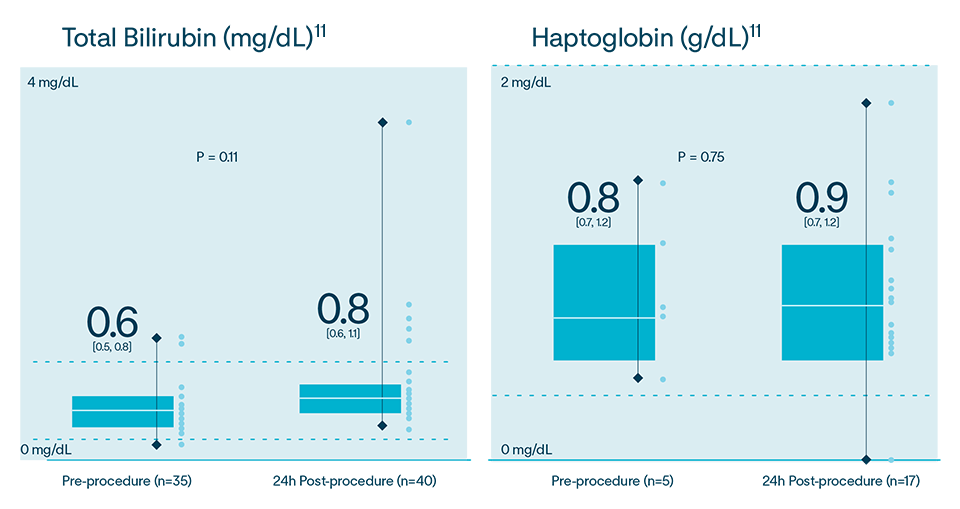
In multiple analyses, no clinically relevant hemolysis was observed, and biomarkers did not exhibit statistically significant change9-11

Hemolysis or kidney injury reported in either VOLT-AF IDE or VOLT CE Mark studies10,11
VOLT-AF IDE Analysis
CE Mark Feasibility Sub-Study Results
In the Volt CE Mark feasibility sub-study, acute effectiveness was achieved in 99.2% (127/128) of treated PVs (96.9% of subjects, 31/32) with 23.8 ± 4.2 PFA applications/subject. No esophegeal lesions causally related to Volt™ PFA System.
Volt AF IDE Study
This clinical research study is intended to demonstrate safety and effectiveness of the Volt™ PFA System for the treatment of symptomatic, recurrent, drug-refractory paroxysmal and persistent atrial fibrillation.
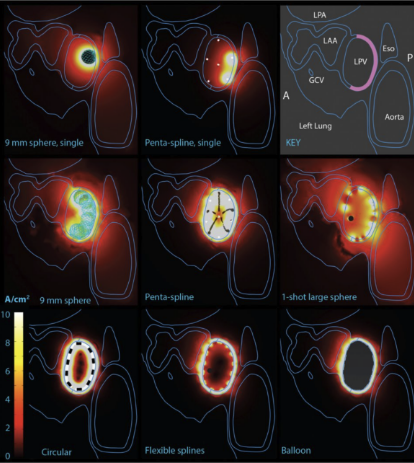
Computer Modeling of PFA Waveform Design
2024 Manuscript Assessing PFA Design Considerations
Comparison of efficiency of PFA catheter designs by computer modeling. Computer models demonstrate a wide range in efficiency among PFA catheters. Form factors such as exposure of PFA electrodes to blood pool significantly influence efficiency. Higher efficiency designs, such as balloon-based designs, have less collateral current.
- Verma, A., et al. (2026, February 5) 12-Month Safety and Effectiveness of a balloon-in-basket PFA system for de novo PVI to treat PAF and PersAF: Results from the VOLT-AF IDE Study [Late Breaking Presentation]. AF Symposium 2026, Boston MA, USA
- Late Breaker presentation at APHRS 2025. Safety and Effectiveness of a Novel Balloon-Based PFA System to Treat PAF and PersAF: 6-Month Results of the VOLT-AF IDE Study (Sanders, et al).
- Sundaram, S. et al. (2025, November 15). Outcomes of Zero-Fluoroscopy Procedures with the Volt PFA Catheter [Short session]. Presented by Prash Sanders. Asia Pacific Heart Rhythm Society (APHRS) 2025, Yokohama, Japan.
- Puererfellner, H. et al. (2025, November). First Real-World Report of Experience with a Novel Balloon-in-Basket Pulsed Field Ablation Catheter [Oral presentation]. Presented by Helmut Puererfellner. Asia Pacific Heart Rhythm Society (APHRS) 2025, Yokohama, Japan.
- Chierchia, G. et al. (2025, November). First Reported Cases of Posterior Wall Ablation Using a Balloon-in-Basket PFA Catheter [Oral presentation]. Presented by Helmut Puererfellner. Asia Pacific Heart Rhythm Society (APHRS) 2025, Yokohama, Japan.
- Tilz, R., et al. (2025, April 26). Long-Term Safety and Effectiveness of balloon-based PFA system for de novo PVI to treat PAF and PersAF: Results from the VOLT CE Mark Study [Oral presentation]. Presented by Prash Sanders. Heart Rhythm Society 2025, San Diego, CA.
- Acute safety and procedural characteristics of conscious and deep sedation to general anesthesia workflows with novel balloon-based PFA system (Oral presentation and abstract by Roland Tilz, EHRA 2025).
- Lo, M., et al. (2025, April 26). Procedural efficiency, safety and acute effectiveness of conscious and deep sedation workflows with novel balloon-based PFA system [Poster presentation]. Heart Rhythm Society 2025, San Diego, CA.
- Marcon L, Della Rocca DG, Vetta G, et al. Hemolysis Biomarkers After Pulmonary Vein Isolation via a Balloon-In-Basket PFA Catheter. Circulation. Online Version of Record before inclusion in an issue. doi:10.1161/CIRCULATIONAHA.124.070333.
- Overmann JA, Marques M, Lafean C, Pipenhagen C, Moon BL, Verma A. Hemolysis Profile of a Novel Balloon-Filled Basket Pulsed Field Ablation Catheter. Poster presented at: AF Symposium 2025; 2025 Jan 15-17; Boston, MA.
- Woods, C., et al. (2025, April 26). Impact of pulsed field ablation using a balloon-in-basket catheter on hemolysis and renal function biomarkers [Poster presentation]. Heart Rhythm Society 2025, San Diego, CA.
- Tilz, R.R. (2025, January 17) Acute results demonstrate safety and effectiveness of balloon-based pulsed field ablation system for de novo PVI in PAF and PersAF [Late Breaking Presentation]. AF Symposium 2025, Boston MA, USA.
- Hemolysis Biomarkers After Pulmonary Vein Isolation via a Balloon-In- Basket PFA Catheter. https://doi.org/10.1111/jce.16608.
MAT-2501234 v10.0