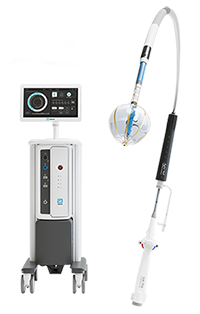
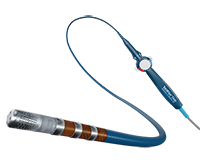
The Volt™ PFA Catheter, Sensor Enabled™, Current™ PFA Generator and TactiFlex™ Duo System are INVESTIGATIONAL DEVICES. LIMITED BY LAW TO INVESTIGATIONAL USE ONLY. These products are not available for sale in the U.S.
Abbott is developing an advanced Pulsed Field Ablation (PFA) portfolio designed to adapt to various clinical scenarios and physician-directed treatments for atrial fibrillation (AFib). This website offers comprehensive information on published clinical study data and pre-clinical testing results that support the safety, efficacy, and long-term effectiveness of Abbott's PFA technologies in treating AFib.
Contact us
Trial questions? Clinical Affairs
Unsolicited requests? Medical Affairs
MAT-2509835 v2.0