Proven Lead Reliability
Independent Fluoroscopic and Electrical Assessment Confirm Durata Lead Reliability1
The Forleo, et al. single-site study was the first systematic fluoroscopic and electrical assessment of Optim™ insulation coated leads (n=413 overall: 225 high voltage, 188 low voltage).
- Average follow-up time was 25.7 +/- 14.1 months.
- During the total follow-up of 10,036 lead-months, there were 7 Optim lead failures.
- Fluoroscopic screening detected no cases of externalized conductors.
Independent Study from VA Hospitals Experience Supports Durata Lead Reliability2
An analysis of more than 4,400 leads with Optim™ lead insulation with up to seven years of follow-up from the VA National Cardiac Device Surveillance Program2 shows:
>99% electrical failure-free survival at five years2
Favorable ICD Lead Survival3
Real-World Survival of Optim™ Leads and Contemporary ICD Leads in Multicenter Study Shows < 0.2% Failure Rate per Year for Durata and Riata ST Optim Leads3
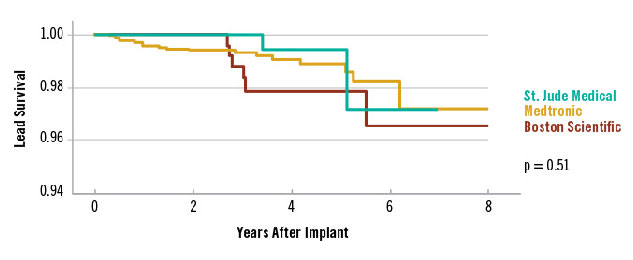
Data on 445 Durata and Riata ST Optim leads from an independent review of 2,653 patients implanted with contemporary ICD leads at four United States clinical centers after a median of 3.2 years of follow-up.3
| Number of leads at risk each year after implant. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| St. Jude Medical Durata and Riata™ ST Optim™ | 445 | 417 | 362 | 262 | 156 | 80 | 31 | 2 | - |
| Medtronic Sprint Quattro Secure‡ | 1821 | 1737 | 1545 | 1177 | 838 | 500 | 232 | 42 | 8 |
| Boston Scientific Endotak Endurance‡ / Endotak Reliance‡ | 389 | 365 | 331 | 257 | 194 | 132 | 79 | 31 | 15 |
Minimizes Lead Abrasion
The Durata lead uses Optim lead insulation, which is supported by evidence from approximately 11,130 leads actively monitored in prospective registries, more than 40,000 patient years of data and multiple third-party publications.4 Postmarket surveillance of these leads demonstrated that there were ZERO reported instances of externalized conductors in the study.5
Clinical Intel: Excellent Long-Term Performance of Abbott ICD Leads Reinforced by ESC Presentation5
- This multi-center prospective study evaluated long-term performance of Durata ICD leads
- A total of 11,132 patients were followed over a period of 11 years – reflecting three prospective registries (beginning in 2006)
- Potential instances of lead failure were adjudicated by an electrophysiologist: There were ZERO reported instances of externalized conductors in the study
- The all-cause mechanical failure rate (0.29%/yr.) reported in this study exceeds industry benchmarks
- Furthermore, the data also includes returned products and non-returns, resulting in an incidence rate that is a true reflection of real-world performance
- This study builds on the earlier publications from HRS, and reinforces our excellent record of long-term ICD lead survival
References
- Forleo GB, Di Biase L, Panattoni G, et al. Systematic fluoroscopic and electrical assessment of implantable cardioverter-defibrillator patients implanted with silicone-polyurethane copolymer (Optim™) coated leads. EP Europace. 2014;16(2):265-270. doi.10.1093/europace/eut236.
- Heart Rhythm Society. Electrical survival analysis of St. Jude Medical Durata and Riata ST Optim high-voltage leads from the VA National Cardiac Surveillance Program. 2016. https://www.abstractsonline.com/pp8/#!/3934/presentation/14474. Accessed November 6, 2018.
- Kramer DB, et al. Transvenous Implantable Cardioverter-Defibrillator Lead Reliability. Journal of the American Heart Association. 2015;4e001672. doi 10.1161/JAHA.114.001672.
- Abbott Laboratories. Abbott Implantable Electronic Systems Product Performance Report. Second Edition, 2023
- Cairns J, Balasubramanian K, Ellison T, Epstein A, Healey J, Connolly S. Low rates of mechanical failures of silicone-polyurethane copolymer- coated ICD leads: 11 years prospective follow-up. Presented at ESC 2019.
MAT-2011141 v2.0
