Long-Term Monitoring for More Effective AF Management: An Assert-IQ™ ICM Product Development Story
Abbott Cardiac Rhythm Management | August 5, 2025
Alex Soriano
Senior Director of Product Development
at Abbott Cardiac Rhythm Management

Behind the Scenes with Abbott Cardiac Rhythm Management
With the increasing prevalence of atrial fibrillation (AF) and the critical need for extended monitoring, the Assert-IQ Insertable Cardiac Monitor (ICM) from Abbott is a key innovation in cardiac care. The Assert-IQ EL+ ICM offers 6+ years* of continuous monitoring without compromise, making it the longest-lasting Bluetooth®-enabled ICM available.1-8 This innovation is meeting the evolving needs of patients and healthcare providers alike.
We sat down with Alex Soriano, Senior Director of Product Development at Abbott Cardiac Rhythm Management, to talk about how this life-changing technology was developed, how its battery was designed to change atrial fibrillation management, and where he and his team would like to take this ICM into the future!
Q: The Assert-IQ ICM is proudly marketed by Abbott as the world’s longest-lasting Bluetooth® functionality ICM “with no compromises.” What exactly does “no compromises” mean?
A: When we designed the overall system, we aimed to be number one in the market. We were mindful that the functionality of competitive ICM systems could be limited in exchange for additional longevity. Saving battery life by limiting patient app settings or using manual Bluetooth® functionality can prevent real-time syncing between the smartphone and device. We designed the Assert-IQ ICM to achieve extended battery longevity, without limiting any functionality or connectivity, enabling continuously monitoring without compromises.
Q: How does the Assert-IQ EL+ ICM 6-year battery longevity compare to other ICMs?
A: There’s no comparison. It is in a league of its own. In published estimations, our technology continues to be very clearly on top overall, while the competition still needs to limit functionality to achieve their maximum labeled longevity.
Q: How does the Assert-IQ EL+ ICM compare in size and implant procedure to others on the market?
A: As always, when developing life-saving technology, the overall workflow of procedure is top of mind. An incision and insertion tool are still used during the procedure. The only difference is a small increase in device thickness – just one millimeter – which does not change the procedure but doubles the battery longevity. The feedback we’ve received from patients is that they’re still comfortable with the device. Overall, one millimeter for an extra three years is a good tradeoff.
Q: What kinds of disciplines within Abbott came together to collaborate on developing the world’s longest-lasting Bluetooth® functionality ICM? Were there any challenges in developing this technology?
A: Every type of discipline came together to overcome the challenges in developing unprecedented technology that could pack more power in a small device. This included expertise in battery chemistry, electrical engineering, mechanical engineering, metal efforts for delicate welds, system engineering for overall architecture, and software counterparts. Some of those team members have been working on their third or fourth master’s degrees in metallurgy while developing the Assert-IQ ICM technology.

Q: It sounds like it truly does not compromise any features for long-term monitoring. What does this mean for long-term monitoring of AF?
A: Some important feedback we’ve received from patients is the peace of mind they feel when they’re continuously monitored. AF can be a chronic challenge. Some patients may need to be monitored pre- and post-ablation. They’re more comfortable when they remain connected to the healthcare system where there is a maintained and uninterrupted workflow in place to help manage their AF. That is what I perceive the Assert-IQ ICM’s longevity means to patients.
Q: Is there any data out there that supports Assert-IQ ICM’s battery performance?
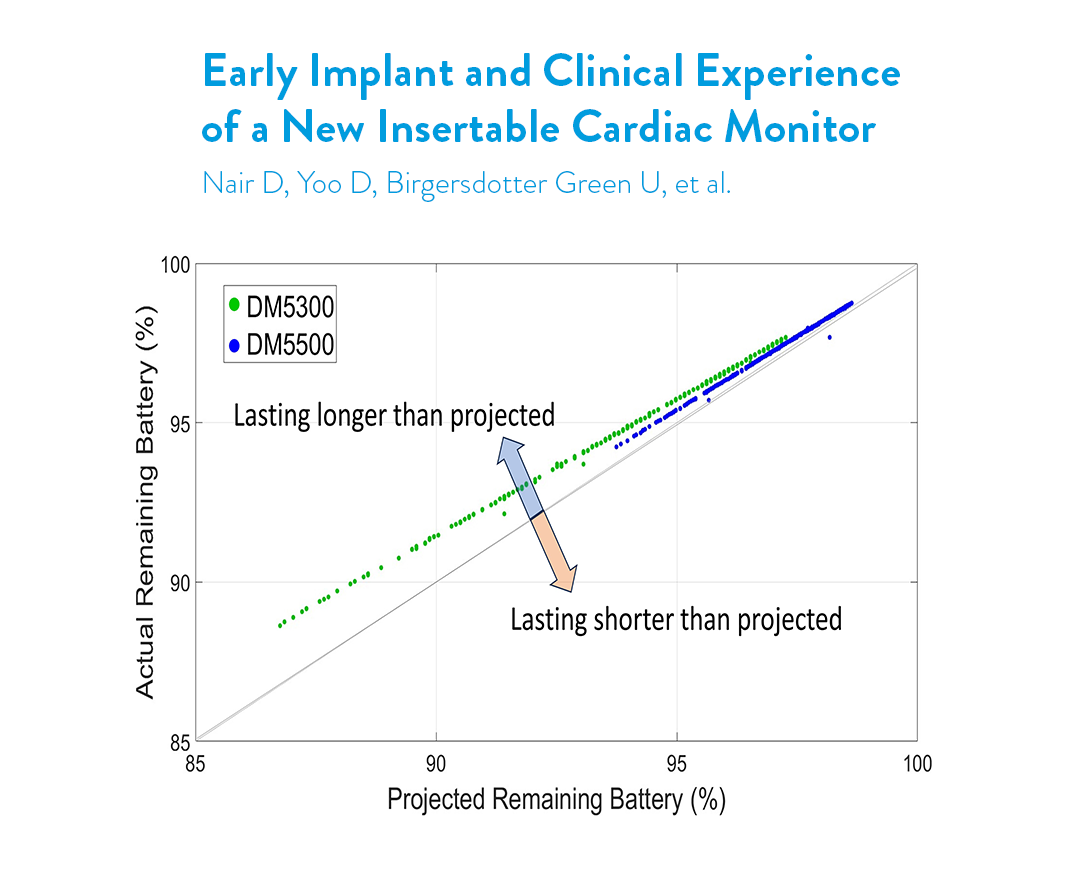
A: Battery longevity data was presented at the 2024 Asia Pacific Heart Rhythm Society (APHRS) Conference. The clinical engineering team randomly selected 600 real-world devices to evaluate diagnostic performance and battery longevity. Results demonstrated that patients indicated for AF management, post-AF ablation, and ventricular tachycardia were twice as likely to be implanted with the 6-year model. The battery analysis for the implanted Assert-IQ ICM devices, with an average follow-up of 65±28 days, showed that 99.8% (599/600 devices) surpassed longevity estimates from our 2023 benchmark testing data.9 You can explore this study and featured presentations and publications on Assert-IQ ICM at our dedicated clinical evidence webpage.
Q: Where do you see insertable cardiac monitoring technology going in the future?
A: It’s incredibly promising. We see the system expanding scope into additional indications. There is potential for ICMs to monitor heart failure and uncontrolled hypertension. When devices can play a big role in early detection, that plays a big role in patient outcomes. We have an incredible platform in the Assert-IQ ICM to build into the future.
Q: How are you leading teams within Abbott to prepare for the future of ICMs?
A: It always starts with the patient. With our primary focus to deeply understand and address unmet clinical needs, we immerse ourselves in the real-world challenges of our customers. That helps engineering develop meaningful solutions throughout the organization. Continuous learning and cross-functional collaboration address the healthcare landscape challenges that are constantly evolving. This is how we deliver exceptional outcomes for patients.
Interested in learning more about the Assert-IQ ICM and its innovative technology for continuous long-term monitoring? Visit the Assert-IQ ICM product page to discover info., clinical evidence and more.
*Data on File, Abbott – Report 90984075
™ Indicates a trademark of the Abbott group of companies.
‡ Indicates a third-party trademark, which is property of its respective owner.
Bluetooth® and Bluetooth® logo are registered trademarks of Bluetooth® SIG, Inc.
References
- Abbott. Assert-IQ™ ICM User Manual.
- Medtronic. REVEAL LINQ‡ LNQ11 Insertable Cardiac Monitor and Patient Assistant PA96000 Clinician Manual. Updated August 26, 2015. Accessed December 31, 2024. https://manuals.medtronic.com/content/dam/emanuals/crdm/ CONTRIB_215651.pdf
- Medtronic. LINQ II‡ LNQ22 Insertable Cardiac Monitor Clinician Manual. Updated September 01, 2022. Accessed December 31, 2024. https://manuals.medtronic.com/content/dam/emanuals/crdm/M032283C001B_view.pdf
- Boston Scientific. User’s Manual, Lux-Dx‡ Insertable Cardiac Monitor System M301, 2925, 2935. Updated July 2020. Accessed December 31, 2024. https://www.bostonscientific.com/content/dam/Manuals/us/current-rev en/92216689-002_LUX-Dx_UM_en_S.pdf
- Boston Scientific. User’s Manual, LUX-Dx II/II+‡ Insertable Cardiac Monitor System M302, M312, 2925, 2929, 2935, 2939. Updated August 2023. Accessed December 31, 2024. https://www.bostonscientific.com/content/dam/elabeling/ crm/51583079-001_LUX-Dx_ICM_UM_en_S.pdf
- Biotronik. Technical Manual BIOMONITOR III‡. Updated December 10, 2020. Accessed December 31, 2024. https:// manuals.biotronik.com/emanuals-professionals/?country=US&product=ImplCardMon/BioMonitor3/BioMonitor3_US
- Biotronik. Technical Manual BIOMONITOR IIIm‡. Updated December 10, 2020. Accessed December 31, 2024. https:// manuals.biotronik.com/emanuals-professionals/?country=US&product=ImplCardMon/BioMonitor3m/BioMonitor3m_US
- Biotronik. Technical Manual BIOMONITOR IV‡. Updated July 17, 2023. Accessed December 31, 2024. https://manuals. biotronik.com/emanuals-professionals/?country=US&product=ImplCardMon/BioMonitor4/BioMonitor4_US
- Nair D, Yoo D, Birgersdotter-Green U, et al. Early Implant and Clinical Experience of a New Insertable Cardiac Monitor. Presented at Asia Pacific Heart Rhythm Society (APHRS); September 26-29, 2024; Sydney, Australia.
Stay Informed
Sign up to hear about our technology, education opportunities, and more.
Read the Latest Blog Article
Stay up to date with recent news, product highlights, and case studies.
MAT-2507273 v1.0