ViewFlex™ X ICE Catheter, Sensor Enabled™ Clinical Studies
The ViewFlex X ICE Study is a prospective, acute, first-in-human, non-significant risk study intended to evaluate a modified version of the ViewFlex X ICE Catheter, SE and ViewMate Multi Ultrasound System ICE data integration with the EnSite X EP System. Outcomes assess physician ability to navigate, and use advanced tools enabled by the ViewFlex X ICE Catheter, SE.
Latest data just released: APHRS 2025
Late-breaking data on the AI-Assist Auto Contour model highlights its design to streamline ICE-guided procedures.1
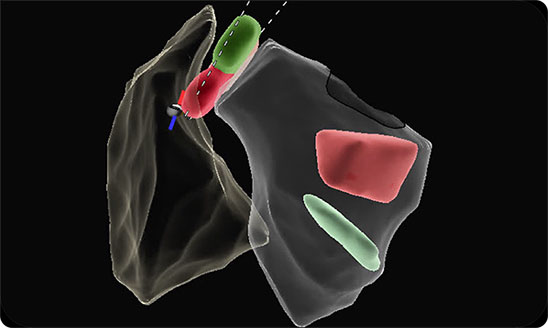
Auto contour powered imaging
Automated segmentation of ICE images matched an expert-drawn data set with high correlation:
~20,000 ICE segments analyzed by ICE experts 20 procedural views


Zero-fluoroscopy workflows
Early results from the ViewFlex X ICE Catheter, SE first-in-human zero fluoroscopy study shows:2
100%
acute procedural success in all cases (N = 92)2
0%
primary safety events among zero-fluoro subjects
Total procedure time
↓ >50%
Lower AF procedure time compared to any fluoro group**
↓ 41%
Lower SVT procedure time compared to any fluoro group**
**Data based on initial observation; sample size (N=12 zero-fluoro AF; N=8 zero-fluoro SVT). No statistical analyses were performed, and the observed differences should not be interpreted as statistically significant.
22%
of subjects treated fluoroscopy-free (N=20/92)

*The DICE coefficienct is a measure of the similarity between two sets.
**Data based on initial observation; sample size (n=12 zero-fluoro AF; n=8 zero-fluoro SVT). No statistical analysis were performed, and the observed differences should not be interpreted as statistically significant.
- Nair, D., Hsu, J. C., Gopinathannair, R., Chinitz, L., Pothineni, N. V., Han, F. T., Dhakal, B. P., Barbhaiya, C., Dave, A., Garcia, F., Wenzel, E., Dahlen, T., Yao, J., Sandler, R. A., Sokol, J. Z., & Winterfield, J. (2025). Accuracy of a Deep Learning Model in Intracardiac Echocardiography. [Oral Late Breaker] Asia Pacific Heart Rhythm Society (APHRS).
- Nair, D., Pothineni, N. V., Dave, A., Valderrabano, M., Hsu, J. C., Gopinathannair, R., Chinitz, L., Han, F. T., Dhakal, B. P., Barbhaiya, C., Gansemer, B., & Winterfield, J. (2025). Innovative Imaging: Fluoroscopy-Free Procedures Using a Novel Integrated Intracardiac Echocardiography Catheter. [Poster presented] Asia Pacific Heart Rhythm Society (APHRS).
Important Safety Information
Indications: The EnSite™ X EP System is a suggested diagnostic tool in patients for whom electrophysiology studies have been indicated. The EnSite™ X EP System provides information about the electrical activity of the heart and displays catheter location during conventional electrophysiological (EP) procedures. Warnings: For patient safety, any connections that directly connect the patient to the EnSite™ X EP System must be routed through the appropriate modules: EnSite™ X EP System SurfaceLink Module, EnSite™ X EP System 20 pin Catheter Input Module, EnSite™ X EP System 80-pin Catheter Input Module and Direct Connect Ports on the EnSite™ X EP System Amplifier. When using the EnSite™ X EP System, full protection against the effects of cardiac defibrillator discharge and other leakage currents is dependent upon the use of appropriate cables. Refer to the ablation catheter IFU for a listing of adverse events related to the use of this device in conjunction with ablation, as a part of the diagnosis and treatment of cardiac arrhythmias. Non-SE catheters cannot collect location data and should not be used for navigation in VoXel Mode because they do not have a magnetic sensor. However, they can be visualized and display intracardiac signals. Only connect items that have been specified as part of the EnSite X EP System or compatible with the EnSite X EP System to the multiple socket-outlets. The EnSite™ X EP System model display should be used in conjunction with conventional EP techniques to confirm catheter location. The AutoMark feature does not indicate lesion effectiveness. AutoMarks are placed based on user-defined parameters for catheter stability and RF metrics only. PFA AutoMarks are placed based on electrode location and user-defined PFA metrics only. Sudden impedance changes of the body or catheter electrodes caused by the connection of other devices (e.g., stimulator, defibrillator, and other devices) may create a location shift. The 2D and 3D LivePoint Displays should not be used as the primary / sole display of tissue proximity during an Electrophysiology study. Refer to the Current PFA Generator IFU for warnings related to the Volt ™ LivePoint Display. Precautions Ensure that surface electrodes, Patient Reference Sensors, and associated connectors do not contact one another, electrical ground, or metallic objects. Do not operate the EnSite™ X EP System Field Frame within 10 m of another operating Field Frame. Do not place the EnSite™ X EP System Field Frame Cable inside the measurement volume or wrap it around the EnSite™ X EP System Field Frame, as it may create a magnetic interference. Metallic equipment used in close proximity to the magnetic field during the procedure, such as a sterile drape holder, may cause metal distortion. Do not place tool cables within 30 mm of the EnSite™ X EP System Field Frame Cable. If placed this close-particularly if the cables are parallel to each other the tool cable may become subject to electromagnetic interference.Do not use the EnSite™ X EP System for magnetic catheter localization or magnetic data collection (NavX SE or EnSite VoXel point collection) if other magnetic fields are present.
Indications: The ViewFlex™ X ICE Catheter Sensor Enabled™ is indicated for use in adult and adolescent pediatric patients for intra-cardiac and intra-luminal visualization of cardiac and great vessels anatomy and physiology, as well as visualization of other devices in the heart. When used with a compatible three-dimensional mapping system, the catheter provides location information. The ViewFlex™ X ICE Catheter Sensor Enabled™ is contraindicated if there is an occurrence of conditions which create unacceptable risk during catheterization. If the patient has a mechanical tricuspid valve (a prosthetic tissue valve is permissible). in patients with an active systemic infection as this may increase the risk for cardiac infection. when there is a presence of deep venous thrombosis or abnormalities or when there is no adequate vascular access. for use in the left side of the heart for patients unable to receive heparin, or an acceptable alternative, to achieve adequate anticoagulation. when there is a presence of a known intracardiac thrombus in a chamber, other chambers are open to use, and via the transseptal approach in patients with left atrial thrombus or myxoma, or interatrial baffle or patch. in the coronary vasculature due to risk of damage to the coronary arteries. The ViewFlex™ X ICE Catheter Sensor Enabled™ is to be used only with the ViewFlex™ Catheter Interface Module and the ViewMate™ Multi ultrasound consoles. Any other use or inappropriate electrical connection may pose a serious risk to patient safety. The ViewFlex™ X ICE Catheter Sensor Enabled™ includes a 9 F shaft. The physician should consider anatomical size restrictions if considering use of the ViewFlex™ X ICE Catheter Sensor Enabled™. Do not use excessive force to advance or withdraw the catheter. Using excessive force can result in patient injury or death. Ensure that the two steering knobs are in the neutral position before advancing or withdrawing the catheter. If you encounter strong resistance during catheter articulation, discontinue the procedure. Identify and address the cause of the resistance before resuming the procedure. Withdraw and redirect the catheter as needed. Have antiarrhythmic drugs, an external defibrillator, and respiratory assist equipment available in case of complications during the use of this device. Catheter materials are not compatible with magnetic resonance imaging (MRI). Arrhythmia, bleeding, cardiac Perforation, cardiovascular injury, cerebrovascular injury, electric shock, embolism, immunological reaction, infection, myocardial ischemia, organ injury, peripheral vascular injury, respiratory compromise.
Indications: ViewMate™ Multi Ultrasound System is applicable for adults, pregnant women, pediatric patients and neonates. It is intended for use in Abdominal, Intra-operative (abdominal, thoracic, and vascular), Pediatric, Small Organ (Thyroid, Breast, Testes), Neonatal Cephalic, Adult Cephalic, Musculo-skeletal (Conventional, Superficial), Cardiac Adult, Cardiac Pediatric, Trans-esoph. (Cardiac), Intra-cardiac, and Peripheral vessel exams. Modes of operation include: B, M, PWD, CWD, Color Doppler, Amplitude Doppler, Combined mode (B+M, PW+B, Color+B, Power+B, PW+Color+B, Power+PW+B), Tissue Harmonic Imaging, TDI, Color M. This device is a general purpose diagnostic ultrasound system intended for use by qualified and trained healthcare professionals for ultrasound imaging, measurement, display and analysis of the human body and fluid, which is intended to be used in a hospital or medical clinic. Contraindications: The diagnostic ultrasound system is not intended for ophthalmic use. Warnings: Do not use an aftermarket probe other than those specified by Mindray. The probes may damage the system causing a profound failure, e.g. a fire in the worst case. Reference manual for full list of warnings. Precautions: This system must be used only by qualified medical professionals. Reference manual for full list of precautions.
MAT-2513928 v2.0