TEAM-HF Clinical Trial
The purpose of the TEAM-HF clinical trial is to evaluate the safety and effectiveness of the HeartMate 3™ Left Ventricular Assist Device (LVAD) compared to guideline-directed medical therapy (GDMT) in ambulatory advanced heart failure patients with refractory pulmonary hypertension who are not dependent on intravenous inotropes.
Refractory pulmonary hypertension, as determined by remote pulmonary artery pressure monitoring using the CardioMEMS™ HF System, will be utilized as an objective measure to identify patients at high risk of worsening heart failure.
CAUTION: Investigational device. Limited by Federal (U.S.) law to investigational use as part of the TEAM-HF trial.
Procedural Overview
System Set-Up
This video describes how to inspect and prepare each of the components used in the implant procedure of the HeartMate 3 LVAD.
Setting up the System Controller
This video shows how to set up the system controller for the HeartMate 3 LVAD.
Preparing the HeartMate 3 Pump
This video shows how the surgical team prepares the HeartMate 3 Pump for implantation.
Preparing the Sealed Outflow Graft
This video shows how to prepare the sealed outflow graft, and conveys warnings for what to avoid during this process.
Patient Preparation
This video shows how to prepare the patient for pump implantation.
HeartMate 3 LVAD Implant Procedure Overview
This video demonstrates HeartMate 3 LVAD implantation with commentary from the operating room surgeon, Dr. Chris Salerno from St. Vincent's Hospital.
Creating the Driveline Exit Site and Tunneling the Driveline
This video shows the process for determining the driveline exit site, and tunneling the driveline.
Preparing the Left Ventricular Inflow Cannula Site
This video shows how the surgical team prepares the left ventricular inflow cannula site.
Connecting the Pump to the Apical Cuff
This video shows how to connect the pump to the apical cuff.
Anastomosing the Outflow Graft to the Ascending Aorta
This video shows how to anastomose the outflow graft to the right lateral aspect of the ascending aorta in a way that prevents kinking or compression of the graft when the chest is closed.
De-Airing & Initiating Pump Operation
This video describes how to de-air and initiate pump operation for the HeartMate 3 LVAD.
Closing and Transitioning to the ICU
This video walks through how to close the HeartMate 3 LVAD implantation site as well as how to prepare and transport your patient to the ICU post-LVAD surgery.
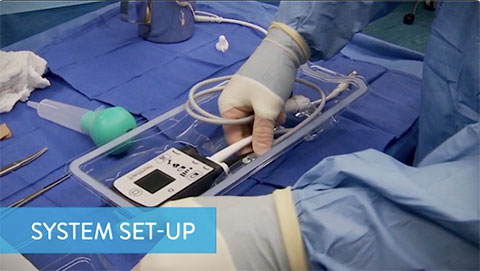
System Set-Up
This video describes how to inspect and prepare each of the components used in the implant procedure of the HeartMate 3 LVAD.

Setting up the System Controller
This video shows how to set up the system controller for the HeartMate 3 LVAD.
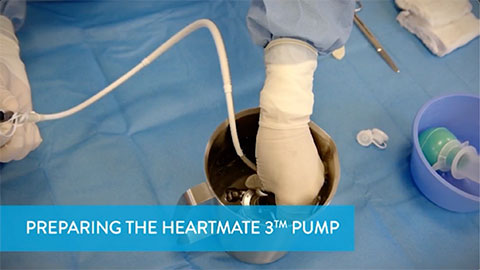
Preparing the HeartMate 3 Pump
This video shows how the surgical team prepares the HeartMate 3 Pump for implantation.
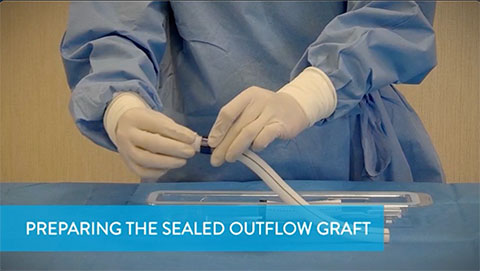
Preparing the Sealed Outflow Graft
This video shows how to prepare the sealed outflow graft, and conveys warnings for what to avoid during this process.
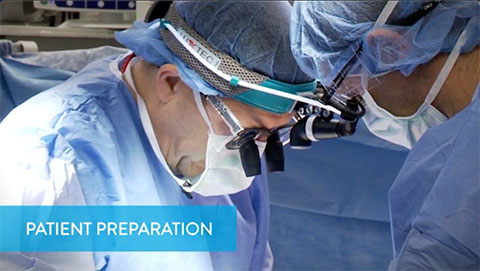
Patient Preparation
This video shows how to prepare the patient for pump implantation.
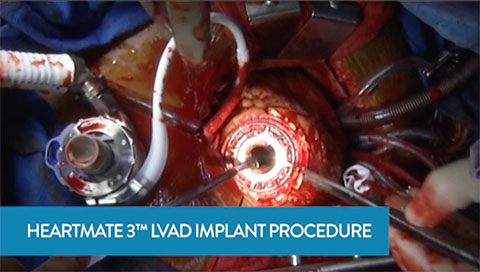
HeartMate 3 LVAD Implant Procedure Overview
This video demonstrates HeartMate 3 LVAD implantation with commentary from the operating room surgeon, Dr. Chris Salerno from St. Vincent's Hospital.

Creating the Driveline Exit Site and Tunneling the Driveline
This video shows the process for determining the driveline exit site, and tunneling the driveline.
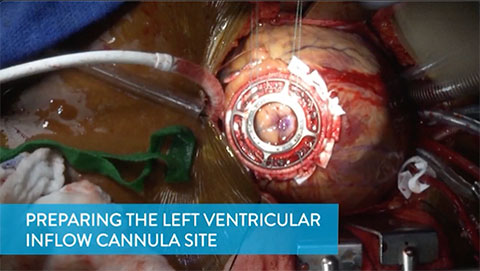
Preparing the Left Ventricular Inflow Cannula Site
This video shows how the surgical team prepares the left ventricular inflow cannula site.
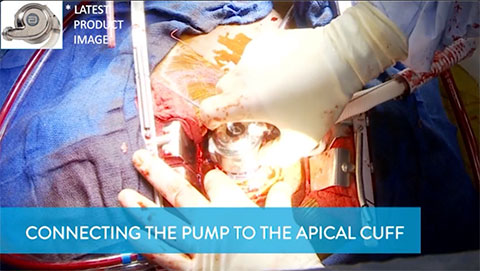
Connecting the Pump to the Apical Cuff
This video shows how to connect the pump to the apical cuff.
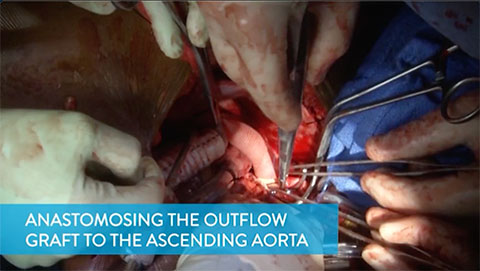
Anastomosing the Outflow Graft to the Ascending Aorta
This video shows how to anastomose the outflow graft to the right lateral aspect of the ascending aorta in a way that prevents kinking or compression of the graft when the chest is closed.
De-Airing & Initiating Pump Operation
This video describes how to de-air and initiate pump operation for the HeartMate 3 LVAD.
Closing and Transitioning to the ICU
This video walks through how to close the HeartMate 3 LVAD implantation site as well as how to prepare and transport your patient to the ICU post-LVAD surgery.
To learn more about the TEAM-HF Trial, including eligibility criteria and study locations, please visit ClinicalTrials.gov website.
Sign Up for Updates
Caution: Investigational device. Limited by federal (or U.S.) law to investigational use as part of the TEAM-HF trial.
CardioMEMS HF System Indications, Safety and Warnings
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
CardioMEMS™ HF System Indications and Usage: The CardioMEMS HF System is indicated for wirelessly measuring and monitoring pulmonary artery pressure and heart rate in NYHA Class II or III heart failure patients who either have been hospitalized for heart failure in the previous year and/or have elevated natriuretic peptides. The hemodynamic data are used by physicians for heart failure management with the goal of controlling pulmonary artery pressures and reducing heart failure hospitalizations.
CardioMEMS HF System Contraindications: The CardioMEMS HF System is contraindicated for patients with an inability to take dual antiplatelet or anticoagulants for one month post implant.
CardioMEMS HF System Adverse Events: Potential adverse events associated with the implantation procedure include, but are not limited to, the following: air embolism, allergic reaction, infection, delayed wound healing, arrhythmias, bleeding, hemoptysis, hematoma, nausea, cerebrovascular accident, thrombus, cardiovascular injury, myocardial infarction, death, embolism, thermal burn, cardiac perforation, pneumothorax, thoracic duct injury or hemothorax.
HeartMate 3 LVAD Indications, Safety and Warnings
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions For Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
HeartMate 3™ Left Ventricular Assist System
Indications: The HeartMate 3™ Left Ventricular Assist System is indicated for providing short- and long-term mechanical circulatory support (e.g., as bridge to transplant or myocardial recovery, or destination therapy) in adult and pediatric patients with advanced refractory left ventricular heart failure and with an appropriate body surface area.
Contraindications: The HeartMate 3 Left Ventricular Assist System is contraindicated for patients who cannot tolerate, or who are allergic to, anticoagulation therapy.
Adverse Events: Adverse events that may be associated with the use of the HeartMate 3 Left Ventricular Assist System are: death, bleeding, cardiac arrhythmia, localized infection, right heart failure, respiratory failure, device malfunctions, driveline infection, renal dysfunction, sepsis, stroke, other neurological event (not stroke-related), hepatic dysfunction, psychiatric episode, venous thromboembolism, hypertension, arterial non-central nervous system (CNS) thromboembolism, pericardial fluid collection, pump pocket or pseudo pocket infection, myocardial infarction, wound dehiscence, hemolysis (not associated with suspected device thrombosis) or pump thrombosis.
HeartMate 3 Coring Tool
Indications: For the HeartMate 3 Left Ventricular Assist System (LVAS) Indications for Use, please refer to the HeartMate 3 LVAS Instructions for Use. The HeartMate 3™ Coring Tool is intended for use with the HeartMate 3 LVAS. The HeartMate 3 Coring Tool provides a means to resect a plug of myocardium from the left ventricle, which allows for HeartMate 3 inflow cannula insertion.
Contraindications: The use of the HeartMate 3 Coring Tool is contraindicated in patients who are contraindicated for HeartMate 3 Left Ventricular Assist System (LVAS) therapy.
Adverse Events: The following adverse events may be associated with the use of the HeartMate 3 Coring Tool. Adverse events are listed in anticipated decreasing order of frequency, except for death, which appears first as it is a non-reversible complication: death, bleeding (perioperative or late), local infection, local ischemia, cardiac arrhythmia, stroke, peripheral thromboembolic event, neurologic dysfunction, hemolysis, sepsis.
CL1027697 Ver B
