TEAM-HF Clinical Trial
The TEAM-HF clinical trial will gather scientific data focusing on the timing of treatments for heart failure (HF). Medicines are usually used to treat advanced HF. Even with these medicines, people can still get sicker over time and need other treatments beyond medicine. One of those treatments is the HeartMate 3™ Left Ventricular Assist Device (LVAD), a heart pump that helps your heart move blood through your body.
This trial will look at whether adding the HeartMate 3™ LVAD to your current treatment can help you live longer and feel better. In addition, the trial will determine if the CardioMEMS™ HF System can help identify people who are at risk for worsening HF based on increased pressure.
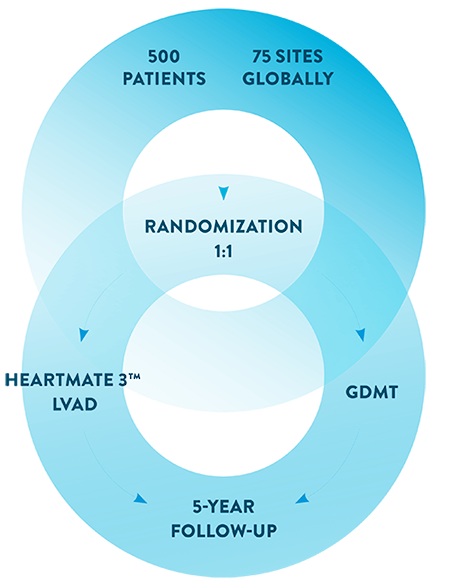
Approximately 500 patients in 75 medical centers around the world will be included in this trial. Half the patients will be randomly assigned to receive a HeartMate 3 LVAD, and the other half will be assigned to standard medical therapy. A qualified team of doctors will monitor patients who participate in the TEAM-HF clinical trial.
Participants will play an important role in helping doctors evaluate the use of the HeartMate 3 LVAD and the CardioMEMS HF System to treat HF patients.
What is Heart Failure?
Heart failure is when the heart has become too weak to pump blood and support your body. Your doctor may refer to heart failure as a ‘weak heart’. Symptoms of heart failure include, but are not limited to:
Tiredness or Fatigue
Constant Coughing
Lightheadedness and Confusion
Trouble Breathing
Swelling
Weight Gain
Faster Heart Rate
Nausea or Lack of Appetite
Symptoms like these can limit your activities and way of life. They may also mean your heart failure is getting worse.
What Are the Stages of Heart Failure?
In its early stages, heart failure can often be managed with medicines and a healthy way of life. As heart failure gets worse and the heart becomes weaker, medicine becomes less effective. Other therapies may be needed.
The New York Heart Association (NYHA) uses “classes” or a heart failure class system to define the seriousness of heart failure and how it progresses over time.7
With each heart failure-related visit to the hospital, the heart function decreases further.
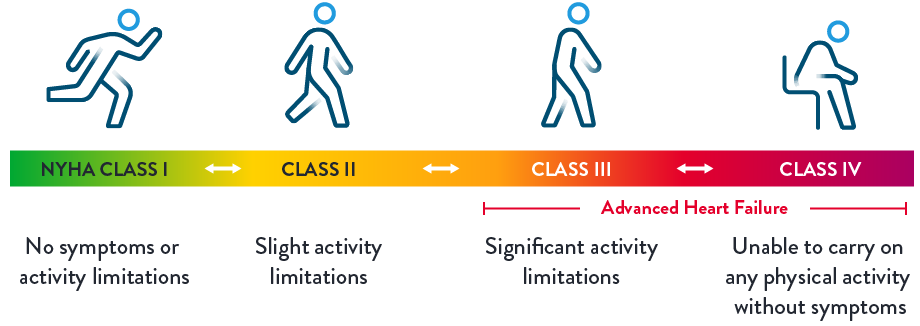
The descriptions above, developed by the NYHA, show each stage and how the related symptoms affect quality of life.
How is heart failure impacting your life?
You can use this tool to:
- identify your heart failure class
- understand the symptoms of heart failure
- talk to your doctor about how it is impacting your life.
Signs of worsening heart failure:
- 2 or more hospitalizations for heart failure
- Increases in dosage of diuretic medications
- The inability to tolerate your heart failure medications (low blood pressure, dizziness, kidney problems)
What are the Treatment Options?
Your doctor will recommend the best treatment for you. There are various treatment options for heart failure, including medications, implantable cardioverter defibrillator, cardiac resynchronization therapy, mitral valve repair, pulmonary artery pressure monitoring and left ventricular assist devices.
Pulmonary Artery Pressure Monitoring
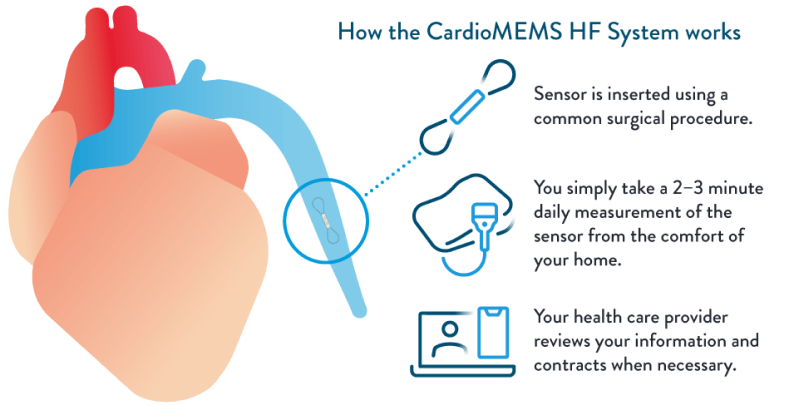
The CardioMEMS HF System monitors the pressure in your pulmonary artery remotely so your doctor can see any changes and make simple adjustments to your care.
What happens during the procedure?
Once you are identified as a candidate for the CardioMEMS™ HF System, you will be scheduled for an implant. During the procedure, the CardioMEMS™ PA Sensor is inserted into the pulmonary artery during a right heart catheterization.
- You may receive mild sedation for comfort.
- A small incision is made, and the catheter is placed into a vein in your neck or groin.
- Guided by X-ray, the catheter is advanced to your heart and into your pulmonary artery.
- Once the catheter is in the correct position, the sensor is released into the artery, where it remains permanently.
- The procedure will take an average of 30-45 minutes and most patients will go home the same day.
As with any medical procedure, there are risks associated with implanting the device, although complications are very rare.
How the HeartMate 3 LVAD Works
An LVAD is a small device that helps pump the blood through the body.
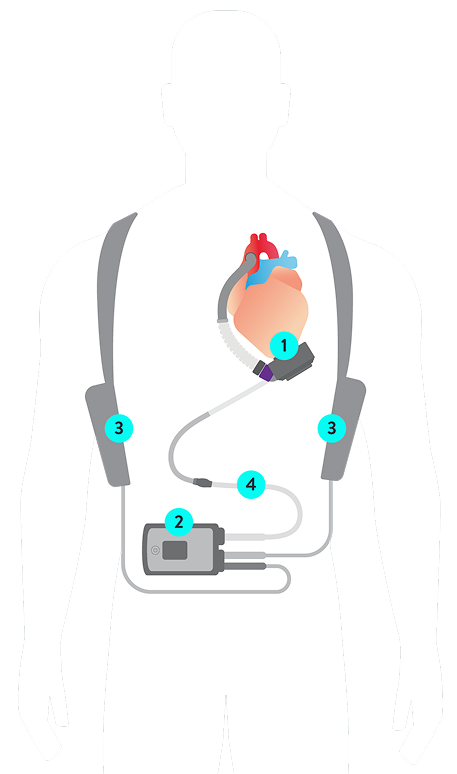
1. HeartMate 3 LVAD
2. System Controller
3. Batteries
4. Modular Driveline
1. HeartMate 3 LVAD
Connected to the left side of the heart and moves oxygenated blood from the left ventricle to the rest of the body.

2. System Controller
Powers and controls the LVAD and is small enough to fit in a pocket. Includes emergency battery backup.

3. Batteries
Provide up to 17 hours of uninterrupted power.

4. Modular Driveline
Facilitates simple replacement of externalized portion.

Mobile Power Unit
Plugs into an electrical socket to provide power while indoors, at rest or asleep. Small, lightweight and mobile, the unit is designed to be extremely durable.


What happens during the procedure?
- The LVAD is implanted by a trained heart surgeon in a hospital surgical procedure that lasts about 4-6 hours.5
- The LVAD is implanted inside your chest. The surgeon then attaches it to the left ventricle of the heart and to the aorta (the main artery that carries blood from your heart to the rest of the body).
- After the LVAD is in place, the surgeon passes a tube (the driveline) through the skin of your stomach area. Then she or he connects it to the controller and a power supply.
- After surgery, you will be moved to a special room called the intensive care unit, or ICU. Here, your doctor and hospital staff can carefully monitor you. You may be in the ICU for a few days.
- After your stay in the ICU, you will be moved to the heart unit for recovery. Your hospital stay can range from 2-4 weeks.
- You and your caregiver will also learn about the care you’ll need after the surgery.
For any questions or concerns you may have regarding the medical procedures, devices and/or your personal health, please discuss these with your physician.
Are you a candidate for the TEAM-HF Trial?
Any gender may participate. You may qualify if:
- You have been diagnosed with heart failure
- You are currently taking medication to treat your heart failure
- You have had more than 1 heart failure hospitalization in last 12 months
What is involved if I choose to participate?
Qualification
Before you are enrolled, your doctor and advanced heart failure team will assess your heart function to make sure your health status qualifies you for the clinical trial.
Evaluation
The first part of the trial will include implantation of the CardioMEMS™ HF System (if you do not already have one) so that your doctor can monitor your pulmonary artery pressure and assess your heart function. If your pressure remains high despite optimal use of medications, you may be eligible for the next step, randomization.
Randomization
If you meet all the requirements, you will be randomized (like flipping a coin) to one of two groups. One group will receive the HeartMate 3™ LVAD, the other group will continue to be treated with medicine. You and your doctors cannot choose which group you will be assigned to.
Follow-Up
If you are assigned to the HeartMate 3 LVAD group, you will need to return to the clinic after three months for a follow-up visit. Regardless of what group you are assigned to, you will need to return to the clinic for follow-up visits at 6 and 12 months. After your first year, you will need to return for visits every year for 5 years.

Frequently Asked Questions
Why should I participate in this trial?
People with advanced heart failure are usually treated with medicine first. However, some patients treated with medicine will still get worse over time. It is hard to know how sick people with heart failure really are because different people show symptoms in different ways.
The TEAM-HF trial wants to find out if there are other ways to know when you might benefit from more than just medications for your heart failure treatment. The study will also find out if an implanted device that pumps blood called the HeartMate 3 LVAD is helpful to treat your heart failure if you are given it sooner.
What will happen if I decide I am interested to take part in this trial?
This research study includes a screening/qualification phase to determine if you are a good candidate for the study. Your trial doctor or other study personnel will ask you medically related questions. Additional tests will be conducted to determine if you qualify for the study. These evaluations will include a physical exam (i.e. height, weight, blood pressure, heart function) and blood test (needle stick to a vein in the arm to withdraw blood).
If you qualify and don’t already have a CardioMEMS™ Pulmonary Artery (PA) Sensor, one will be implanted to take part in this trial. The data obtained from your CardioMEMS HF System will be used by your doctor to adjust your medications.
Once all these tests have been completed, your trial doctor will decide if you qualify to take part in the next phase of the TEAM-HF study and you’ll be assigned to a group (arm). If you do not qualify for the next phase of the study, your participation will end.
Do I have a choice in which study arm treatment I receive?
If qualified for the study, you will not have a choice in what treatment you receive as TEAM-HF is a controlled randomized study.
Randomization is the process by which you will be randomly (by chance) assigned to either of two groups (control group: Medications, or treatment group: HeartMate 3). Randomization is like a coin toss in deciding which group you will be part of. A computer program will decide which group you will be in and you will have equal chance of being assigned to the control group or to the treatment group.
Due to the nature of the treatment, you as well as your doctor will be aware of the treatment assignment.
What happens if I am randomized to the control group?
If you are assigned to the control group, your medical team will continue adjusting your medications based on the pressures from your CardioMEMS PA sensor implant as you take your daily readings.
What happens if I am randomized to the treatment group?
If you are assigned to the treatment group, you will be implanted with a HeartMate 3 LVAD in addition to the CardioMEMS HF System. Your medical team will be closely assessing your heart functions and your pressures by utilizing both devices.
Will the HeartMate 3 LVAD implant require open heart surgery?
If you are assigned to the treatment group, you will have surgery to implant the HeartMate 3 LVAD. One part of the pump will be attached to the bottom of your heart and another part will be attached to your aorta (a large blood vessel that carries blood from your heart to your body). A cable will go from the pump through the skin on your belly to a controller to power the pump. The pump can be powered by two batteries, or a mobile unit that is plugged into an AC wall outlet.
What are the risks associated with this trial?
Being part of any research study comes with risks, discomforts, and difficulties. These risks can also affect an embryo, unborn child, or nursing infant if you become pregnant. It is important to think about these risks carefully. This study will have follow-up tests and procedures. The risks and side effects for each person can be different. Ask the study doctor if you have any questions.
Possible risks include, death, stroke, bleeding, infection. We do not know if the HeartMate 3 LVAD device will help you. There is a chance the HeartMate 3 LVAD could make your heart failure or your health worse.
What type of medication would I take being part of the TEAM-HF study?
Your study doctor will most likely ask you to start taking blood thinning medication after the implant procedure. This medication is important to prevent blood clots and for the HeartMate 3 LVAD to work properly.
Your doctor will discuss your treatment medications with you. Your medications will be customized to treat your heart failure by your medical team.
Will I continue to see my current doctor if I take part in this trial?
If you decide to participate in the trial, you will be managed by a study team which will include a cardiologist trained on the trial (if different from your cardiologist) and your regular cardiologist.
The two will work as a team to provide the best care for you.
What other choices do I have?
You will receive available treatment for heart failure whether or not you take part in this trial.
Your doctor will discuss other treatment options with you if you decide not to join. Other options may include continuing to take medication to treat your heart condition or a heart transplant. One of your treatment options could still be the HeartMate 3 LVAD even if you decide not to join the study. Your doctor will explain in more detail the various alternative procedures or therapies available to you and the risks and benefits of these alternatives.
Your future care and your relationship with your doctor will not be affected if you decide not to be in this trial.
References
- Martin SS, Aday AW, Almarzooq ZI, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee; Stroke Statistics Subcommittee. Circulation. 2024;149:e347–913.
- Setoguchi S, et al. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260-266.
- National Center for Health Statistics. Multiple Cause of Death 2018–2022 on CDC WONDER Database. Accessed October 13, 2024.
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Table 20-2 HF in the United States. Circulation. 2020;141:e139-e596
- Ambardekar AV,, et al. Outcomes with Ambulatory Advanced Heart Failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J Heart Lung Transplant. 2019 Apr;38(4):408–17.
- National Cancer Institute - Cancer trends progress report 2024 https://progressreport.cancer.gov/after/survival. Accessed October 18, 2024.
CardioMEMS HF System Indications, Safety and Warnings
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
CardioMEMS™ HF System Indications and Usage: The CardioMEMS HF System is indicated for wirelessly measuring and monitoring pulmonary artery pressure and heart rate in NYHA Class II or III heart failure patients who either have been hospitalized for heart failure in the previous year and/or have elevated natriuretic peptides. The hemodynamic data are used by physicians for heart failure management with the goal of controlling pulmonary artery pressures and reducing heart failure hospitalizations.
CardioMEMS HF System Contraindications: The CardioMEMS HF System is contraindicated for patients with an inability to take dual antiplatelet or anticoagulants for one month post implant.
CardioMEMS HF System Adverse Events: Potential adverse events associated with the implantation procedure include, but are not limited to, the following: air embolism, allergic reaction, infection, delayed wound healing, arrhythmias, bleeding, hemoptysis, hematoma, nausea, cerebrovascular accident, thrombus, cardiovascular injury, myocardial infarction, death, embolism, thermal burn, cardiac perforation, pneumothorax, thoracic duct injury or hemothorax.
HeartMate 3 LVAD Indications, Safety and Warnings
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions For Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
HeartMate 3™ Left Ventricular Assist System
Indications: The HeartMate 3™ Left Ventricular Assist System is indicated for providing short- and long-term mechanical circulatory support (e.g., as bridge to transplant or myocardial recovery, or destination therapy) in adult and pediatric patients with advanced refractory left ventricular heart failure and with an appropriate body surface area.
Contraindications: The HeartMate 3 Left Ventricular Assist System is contraindicated for patients who cannot tolerate, or who are allergic to, anticoagulation therapy.
Adverse Events: Adverse events that may be associated with the use of the HeartMate 3 Left Ventricular Assist System are: death, bleeding, cardiac arrhythmia, localized infection, right heart failure, respiratory failure, device malfunctions, driveline infection, renal dysfunction, sepsis, stroke, other neurological event (not stroke-related), hepatic dysfunction, psychiatric episode, venous thromboembolism, hypertension, arterial non-central nervous system (CNS) thromboembolism, pericardial fluid collection, pump pocket or pseudo pocket infection, myocardial infarction, wound dehiscence, hemolysis (not associated with suspected device thrombosis) or pump thrombosis.
HeartMate 3 Coring Tool
Indications: For the HeartMate 3 Left Ventricular Assist System (LVAS) Indications for Use, please refer to the HeartMate 3 LVAS Instructions for Use. The HeartMate 3™ Coring Tool is intended for use with the HeartMate 3 LVAS. The HeartMate 3 Coring Tool provides a means to resect a plug of myocardium from the left ventricle, which allows for HeartMate 3 inflow cannula insertion.
Contraindications: The use of the HeartMate 3 Coring Tool is contraindicated in patients who are contraindicated for HeartMate 3 Left Ventricular Assist System (LVAS) therapy.
Adverse Events: The following adverse events may be associated with the use of the HeartMate 3 Coring Tool. Adverse events are listed in anticipated decreasing order of frequency, except for death, which appears first as it is a non-reversible complication: death, bleeding (perioperative or late), local infection, local ischemia, cardiac arrhythmia, stroke, peripheral thromboembolic event, neurologic dysfunction, hemolysis, sepsis.
CL1027697 Ver B
