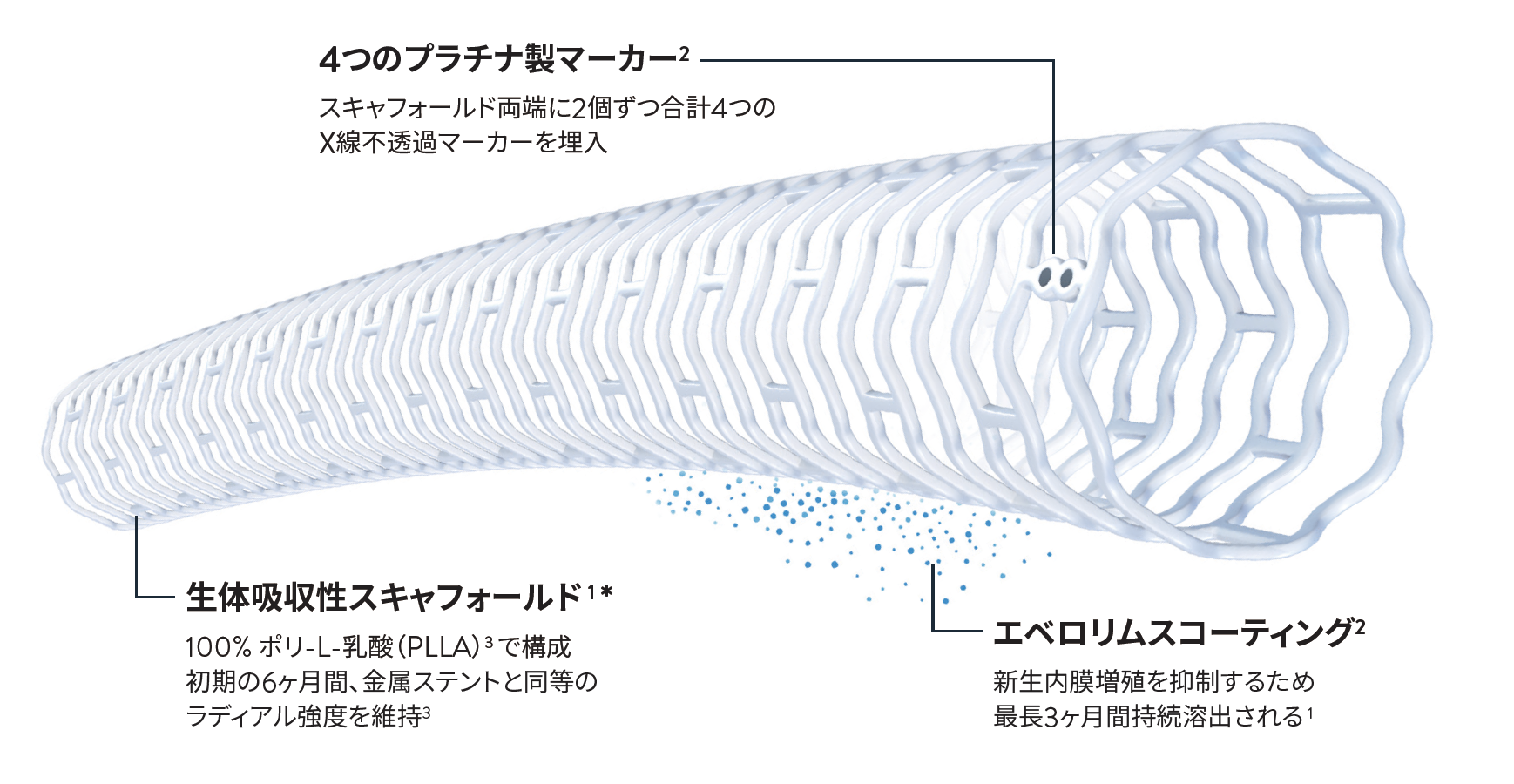
Esprit™ BTKは生体吸収され、将来的に LEAVES NOTHING BEHINDを実現します 1 *
Esprit™ BTK 生体吸収過程
スキャフォールドが血管に留置され、薬剤が徐放された後、スキャフォールドは経時的に分解され、スキャフォールドマーカーを除いて生体吸収される 1 *
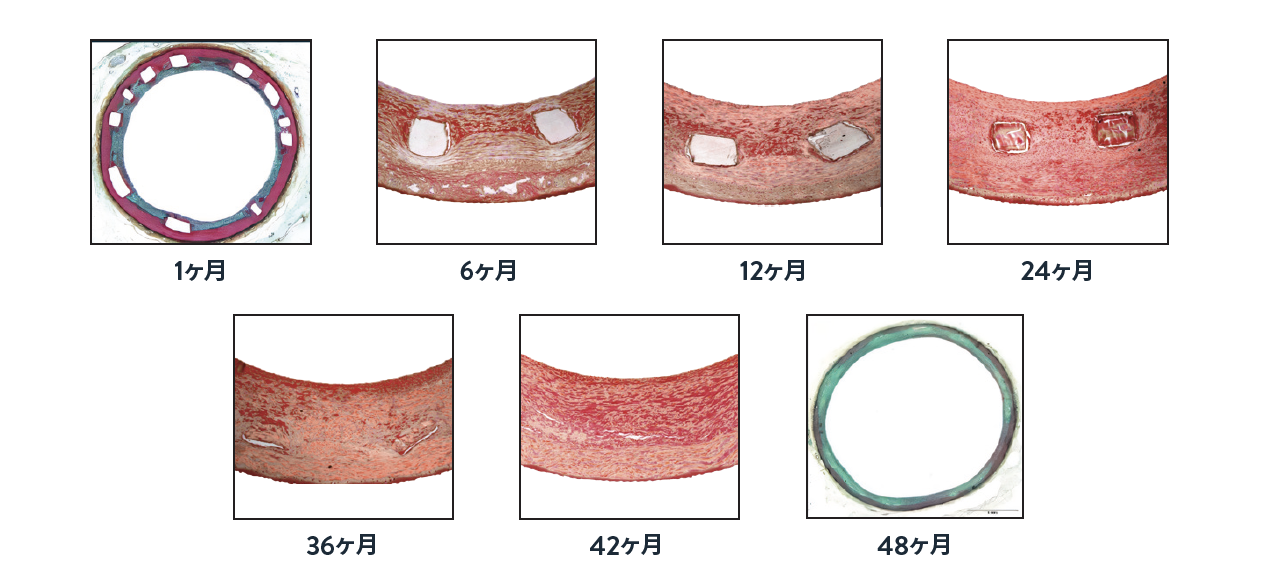
Absorb™ BVS を留置したブタ冠動脈の代表的な顕微鏡写真( 12 ~ 48ヶ月)
* プラチナ製マーカーを除く
References
- Data on file at Abbott.
- Esprit™ BTK Everolimus Eluting Resorbable Scaffold System Instructions for Use (IFU).
- Data on file at Abbott. Testing done with XIENCE Sierra™ 3.5 x 38 mm at nominal.
- Brian G. DeRubertis et al., Two-Year Outcomes of the LIFE-BTK Randomized Controlled Trial Evaluating the Esprit™ BTK Drug-eluting Resorbable Scaffold for Treatment of Infrapopliteal Lesions, VIVA 2024.
- Superiority analysis was performed at 1 year.
- By Newcombe score method.
- Varcoe, R., Primary Outcomes of the Esprit™ BTK Drug-Eluting Resorbable Scaffold for the Treatment of Infrapopliteal Lesions: The LIFE-BTK Trial. Presented at TCT 2023.
- Reintervention defined as CD-TLR.
MAT-2507542 v1.0
