Platform Matters—Unique Interwoven Design of Supera™ Stent
Breakthrough Technology in Stent Platform
The Supera™ Stent’s unique design results in a platform with features unmatched among standard nitinol peripheral stents:
Strength—its high resistance to compression1
Low chronic outward force against vessel walls2
High flexibility,3 which allows for fracture resistance4
View the Supera™ Stent Difference
The interwoven nitinol design provides high structural integrity and kink resistance, producing the features that set the Supera™ Stent apart.
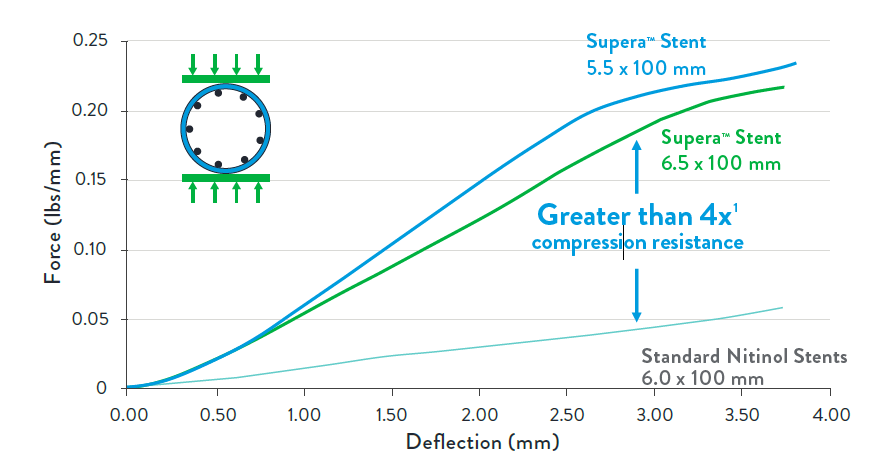
Offering Unparalleled Strength for Compression Resistance
The Supera™ Stent exhibits more than 4 times the compression resistance of all other self-expanding nitinol stents.2
Optimizes luminal gain and maintains circular geometry
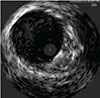
SUPERA™ STENT21
Same artery shown, with a Supera™ Stent distal to the SNS
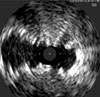
SNS21
Exerting the Least Chronic Outward Force on the Vessel Wall2
While laser-cut nitinol stents are designed and required to be oversized for deployment, the Supera™ Stent is unique in that it is sized 1:1 with the vessel diameter. So as seen in the image below, the laser-cut stents inherently exert force on the vessel wall throughout the lifetime of the stent, which in turn can result in endovascular injury over the long term. The lower chronic outward force (COF) with the Supera™ Stent results in fewer vessel injuries.2,4
Standard Nitinol Stent (SNS) Designs
 | Pushes against the vessel to open, through lifetime of SNS |  | 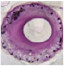 |
| STENT OVERSIZING | HIGH COF2 | VESSEL INJURY4 | |
| Chronic outward force (COF) is exerted on vessel by self-expanding stents due to inherent oversizing | |||
Supera™ Stent
 | 1:1 sizing allows to scaffold the vessel to maintain open |  |  |
| SIZING 1:1 | LOW COF2 | MINIMAL VESSEL INJURY4 | |
| Supera™ Stent is sized 1:1 with the prepared vessel and therefore has minimal chronic outward force | |||
Providing Unparalleled Flexibility3 and Zero Fractures4-20
Other self-expanding nitinol stents are fashioned from a rigid, inflexible tube that is laser-cut. As shown in the photo below, due to design and materials properties, some laser cut stents are less likely to conform to a dynamic vascular environment and can potentially kink and fracture in tortuous vessels (left).
Laser-Cut Stents

Supera™ Stent

With the Supera™ Stent, however, individual flexible nitinol wires are interwoven for unparalleled flexibility,3 excellent kink resistance, and the ability to mimic the natural movement of the anatomy1,21,22 (right).
Consequently, data on over 2,000 patients published in 17 studies have shown that at 1 year the Supera™ Stent has zero fractures.4-20

REFERENCES
- Data on file at Abbott.
- Competitors tested include Astron Pulsar-18, Complete SE, EverFlex, Innova, LifeStent, Misago, S.M.A.R.T., and Zilver PTX. Data on file at Abbott.
- Flexibility is defined as kink resistance. Competitors tested include Astron Pulsar-18, Complete SE, EverFlex, Innova, LifeStent, Misago, S.M.A.R.T., and Zilver PTX. Data on file at Abbott.
- Garcia L. et al., Catheterization and Cardiovascular Interventions 2017 Jun 1;89(7):1259-1267.
- Brescia AA. et al., J Vasc Surg. 2015 Jun;61(6):1472-8
- Chan YC. et al., J Vasc Surg. 2015 Nov;62(5):1201-9.
- Dumantepe M. Vasc Endovascular Surg. 2017 Jul;51(5):240-246.
- George JC. et al., J Vasc Interv Radiol. 2014 Jun;25(6):954-61.
- Goltz JP. et al., J Endovasc Ther. 2012 Jun;19(3):450-6.
- León LR Jr. et al., J Vasc Surg. 2013 Apr;57(4):1014-22.
- Montero-Baker M. et al., J Vasc Surg. 2016 Oct;64(4):1002-8.
- Myint M. et al., J Endovasc Ther. 2016 Jun;23(3):433-41.
- Palena LM. et al., J Endovasc Ther. 2018 Oct;25(5):588-591.
- Scheinert D. et al., JACC Cardiovasc Interv. 2013 Jan;6(1):65-71.
- Scheinert D. et al., J Endovasc Ther. 2011 Dec;18(6):745-52.
- Steiner S. et al., J Endovasc Ther. 2016 Apr;23(2):347-55.
- Werner M. et al., EuroIntervention. 2014 Nov;10(7):861-8.
- San Norberto EM. et al., Ann Vasc Surg. 2017 May;41:186-195.
- Teymen B. et al., Vascular. 2018 Feb;26(1):54-61.
- Bhatt H. et al., Cardiovasc Revasc Med. 2018 Jul;19(5 Pt A):512-515.
- Arena F. et al., J Vasc Med Surg. 2013:1;116.
- Chen Y. et al., J Vasc Surg 2014;59:384-91.
MAT-2106533 v2.0

