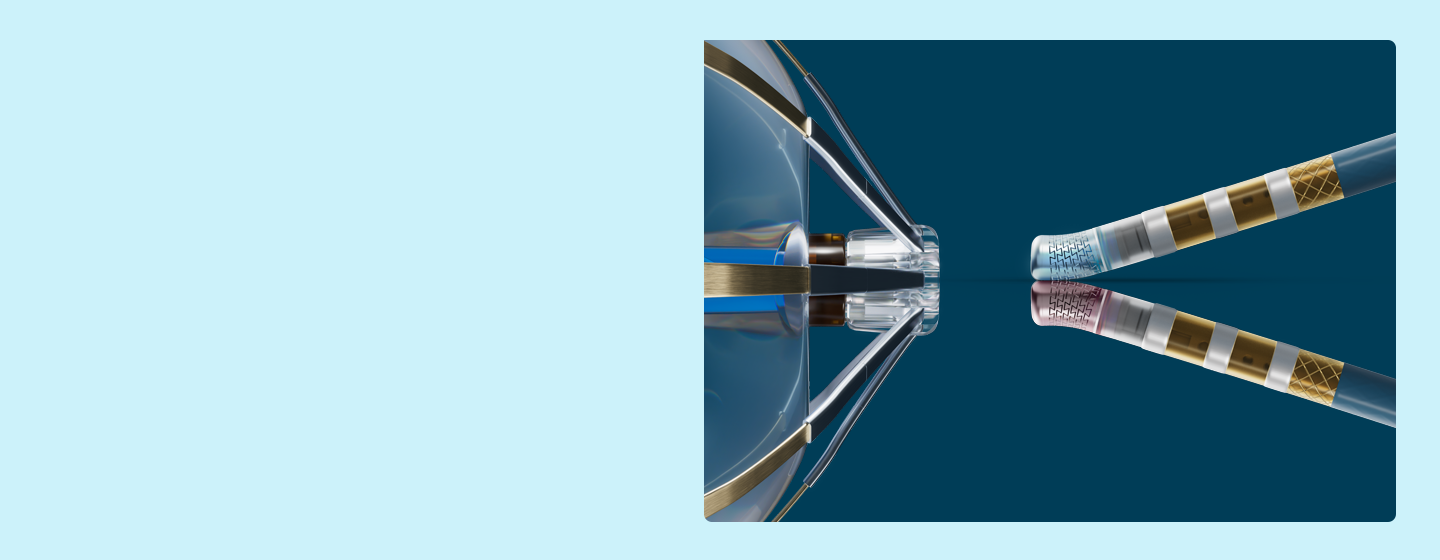
You and your patients deserve more than ordinary.
Every tool we create is built with a comprehensive focus on pulsed field ablation (PFA) science and your clinical workflows - empowering you to tailor treatment for each patient.
Uniquely engineered with the single goal of giving you confidence in safety, efficacy, and efficiency, our suite of PFA solutions is backed by the most comprehensive ecosystem in the industry.
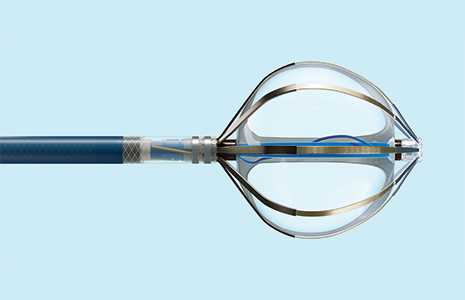
Volt™ PFA System
Reengineering PFA with minimal applications1 and total control.
Volt PFA System simplifies therapy delivery while minimizing procedural burden1,2 so you can treat more patients with ease and precision.1*
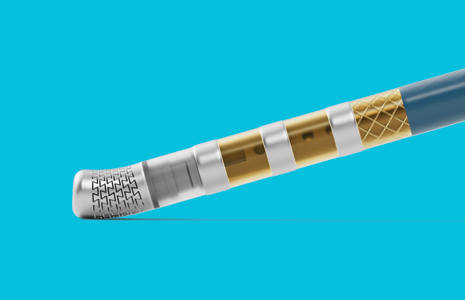
TactiFlex™ Duo Ablation Catheter, Sensor Enabled™
Intentionally built to provide the versatility, precision, and freedom necessary to confidently tailor therapy,3 switch seamlessly between PF and RF energy,4 and achieve consistent, predictable lesions5– every time.
References
* Faster procedures allow us to treat more patients.
1. Tilz, R.R. (2025, January 17) Acute results demonstrate safety and effectiveness of balloon-based pulsed field ablation system for de novo PVI in PAF and PersAF [Late Breaking Presentation]. AF Symposium 2025, Boston MA, USA.
2. Acute safety and procedural characteristics of conscious and deep sedation to general anesthesia workflows with novel balloon-based PFA system (Oral presentation and abstract by Roland Tilz, EHRA 2025).
3. Sanders, P. et al. (2025, November 13). Beyond Pulmonary Veins: Ablation Trends and Targets with a Novel Flexible-Tip Dual-Energy PF/RF Ablation Catheter [Oral presentation]. Presented by Prash Sanders. Asia Pacific Heart Rhythm Society (APHRS) 2025, Yokohama, Japan.
4. Data on file 91062308.
5. Friedman, et al. (2025 September) Development of a PFA index to guide energy delivery with a force sensing flexible, irrigated tip catheter [Oral presentation]. ESC 2025, Madrid, Spain.
MAT-2600837 v1.0