TactiFlex™ Duo Ablation Catheter, Sensor Enabled™ is available for sale in EUROPE.
Caution: investigational device. Limited by Federal (or United States) law to investigational use.
TactiFlex™ Duo System
The PFA components of the system consist of the following:
- TactiFlex™ Duo Ablation Catheter, Sensor Enabled™: A novel dual energy, flexible-tip focal ablation catheter that can transmit either RF energy or PFA therapy. It is designed for therapy delivery, pacing, and collection of electrical and anatomical data when used with the EnSite™ X EP System.
- Current™ PFA Generator: The streamlined user interface includes energy source selection and PFA waveform selection. Abbott’s Current PFA Generator is designed for intuitive use and can also be used with the Volt™ PFA Catheter, Sensor Enabled™.
The RFA components of the system consist of the following:
- Ampere™ RF Generator
- CoolPoint™ Pump
- TactiSys™ Quartz Equipment
Latest data released at AF Symposium 2026
6-month data evaluated safety and effectiveness in a range of conditions1
Recent results from the FOCALFLEX Study describe the use of the TactiFlex Duo System in pulsed field ablation procedures and outline observations related to safety data and ablation approaches evaluated in the study.
6-month findings evaluate strong clinical performance across multiple dimensions:
1.4%
Primary Safety Endpoint (2/147)
81%
of patients remained free from documented arrhythmia recurrence at 6 months (AF/AFL/AT)
1
Repeat ablation (1/144) after the blanking period
Lesion strategy distribution
79.2%
treated with PF-only lesions
20.1%
treated with combined PF + RF
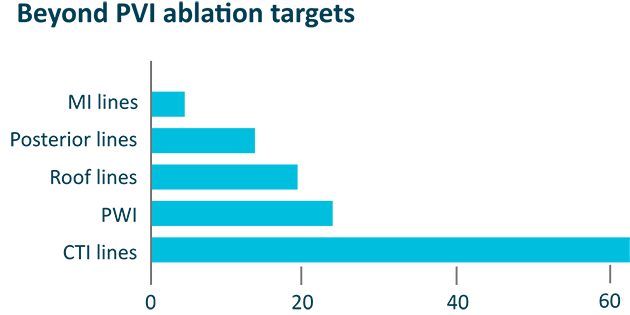
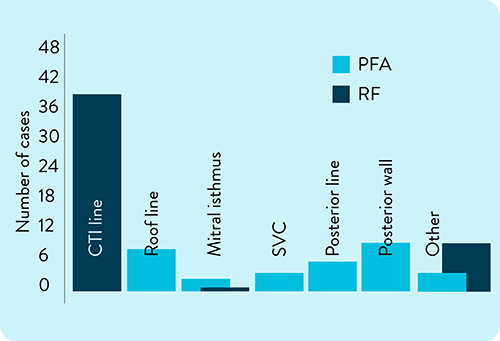
Duo catheter used beyond the PVs in 20.8% of patients, including:
CTI line (18/144); Posterior wall (12/144); Roof line (9/144)
Quality of life improvements:
AF Effect on Quality of Life (AFEQT) overall score: +22.0
EQ-5D-5L Visual Analog Score (VAS): +6.6
Data released at APHRS/JHRS 2025
Flexible sedation strategies2
Acute success was evaluated under deep sedation (DS) or general anesthesia (GA) with TactiFlex Duo Ablation Catheter, SE:
No significant differences in acute safety or effectiveness across sedation groups
Skeletal muscle recruitment remained clinically acceptable2
100%
Acute success in the DS group2
99.6%
Acute success in the GA group2
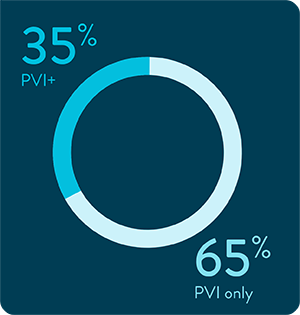
Expanding beyond PVI: ablation across non-PV targets3
TactiFlex Duo Ablation Catheter, SE in scenarios beyond PVI. In early clinical experience:
29.9%
of subjects (n = 97/324) received ablation beyond PVI3
0%
coronary spasm reported in the PVI+ group3
1%
primary safety events reported in the PVI+ group3
Clinical evidence
Before being used with patients, the TactiFlex™ Duo System, underwent extensive laboratory and pre-clinical testing. Following this, two clinical trials were initiated:
FOCALFLEX CE Mark Trial4
Effectiveness



Safety

Procedural information
Total PV ablation time:
Median (Q1,Q3): 43.0 (36.0, 55.0) min
Total PFA applications for PVI:
93.5 ± 32.9 PFA applications/patient
Sedation method
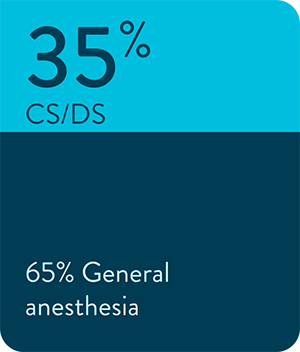
Energy type used for PVI

Demographics

FlexPulse IDE Study5
This clinical research study is intended to demonstrate safety and effectiveness of the TactiFlex™ Duo System and Current PFA Generator for the treatment of symptomatic, recurrent, drug refractory paroxysmal atrial fibrillation.
View FlexPulse IDE Study Design
Demographics
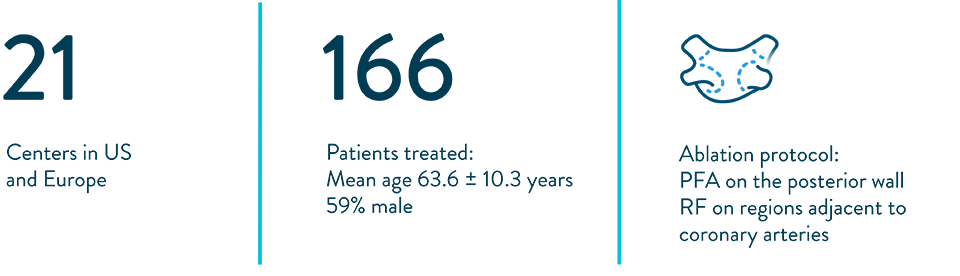
Effectiveness
100%
Acute effectiveness
(n=166/166)
Procedural Information
Total PV ablation time:
Median (Q1,Q3): 45.5 (35.0, 60.0) min
Total PFA applications for PVI:
101.9 ± 26.0 PFA applications/patient
Sedation method

Energy type used for PVI

Ablation target
Beyond PVI ablation targets
Pre-clinical data
2025 HRS Abstract: Chronic Canine Safety & Efficacy6
Demonstrates the safety and effectiveness of PF and RF+PF lesions delivered using the TactiFlex Duo System catheter in creation of focal, linear, and circumferential atrail lesion sets using two distinct PFA waveforms.
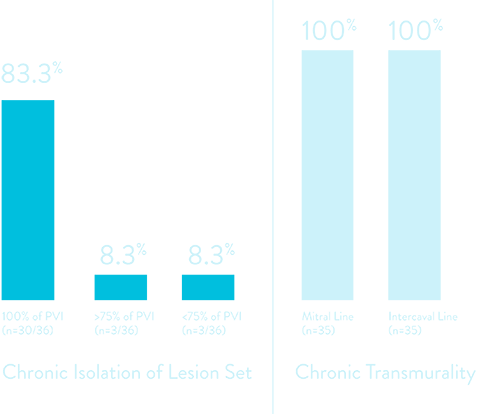
* PVI confirmation after 20-minute wait period
** After 20-minute wait period and no early reconnections
References:
- Silberbauer, J., et al. (2026, February 5) Safety and Effectiveness of the TactiFlex Duo System: 6-Month Results of the FOCALFLEX Study [Late Breaking Presentation]. AF Symposium 2026, Boston MA, USA.
- Scherr, D. et al. (2025, November). General anesthesia vs. Deep sedation and Conscious Sedation in Subjects Undergoing Pulmonary Vein Isolation with a Novel Flexible-Tip Dual-Energy PF/RF Ablation Catheter [Oral Presentation]. Presented by Daniel Scherr. Asia Pacific Heart Rhythm Society (APHRS) 2025, Yokohama, Japan.
- Sanders, P. et al. (2025, November 13). Beyond Pulmonary Veins: Ablation Trends and Targets with a Novel Flexible-Tip Dual-Energy PF/RF Ablation Catheter [Oral presentation]. Presented by Prash Sanders. Asia Pacific Heart Rhythm Society (APHRS) 2025, Yokohama, Japan.
- Silberbauer, J., et al (2025, April 25). Initial Clinical Experience with the TactiFlex DUO System: Safety and Acute Results of the FOCALFLEX Study
[Oral presentation]. Presented by Daniel Scherr. Heart Rhythm Society 2025, San Diego, CA. - Lo, M., et al (2025, April 26). Acute Results of the FlexPulse IDE Trial [Poster presentation]. Heart Rhythm Society 2025, San Diego, CA.
- Koruth, J. et al. Acute and chronic pulmonary vein isolation durability and safety assessments of a focal dual-energy, flexible 4mm contact-sensing tip [Poster presentation]. Heart Rhythm Society 2025, San Diego, CA.
Rx Only. Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events, and directions for use.
MAT-2505447 v5.0