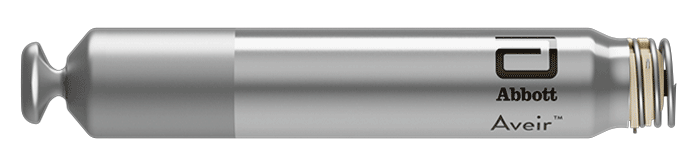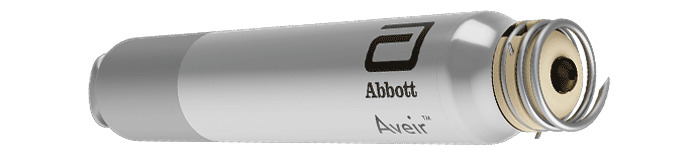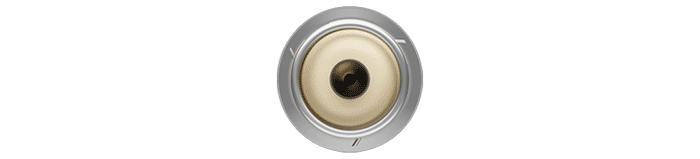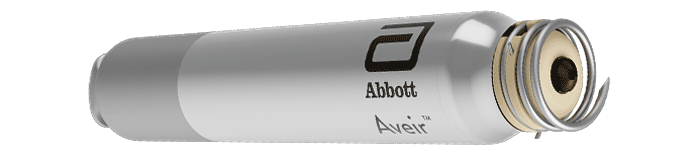
AVEIR™ VR Ventricular
Leadless Pacemaker
Advancing management of bradycardia one beat at a time
Tailored Leadless Pacing
AVEIR VR Ventricular Leadless Pacemaker (LP) is the next evolution in leadless technology that is ideal for patients requiring single chamber pacing for bradycardia or other rhythm disorders.1 AVEIR VR LP has been designed for:
- long-term retrieval2
- an extended battery life
- electrical mapping prior to fixation for optimal device location
- providing an upgradeable platform to later support a dual chamber pacing system when patient therapy need evolves3
Designed for Long-Term Retrieval2
The AVEIR VR LP's predecessor has an overall long-term retrieval success rate above 88% with helix fixation through 9 years of retrieval experience. Limited data is available for AVEIR VR LP.
The AVEIR VR LP's predecessor has an overall long-term retrieval success rate of 92.5% for implants ≥ 5 years.
Long-Lasting
Increased projected battery longevity over current available leadless pacemakers3,4 opens the door to more patients.
Mean ± SD: 17.6±6.6 years5
95% CI: 16.6 to 18.6 years
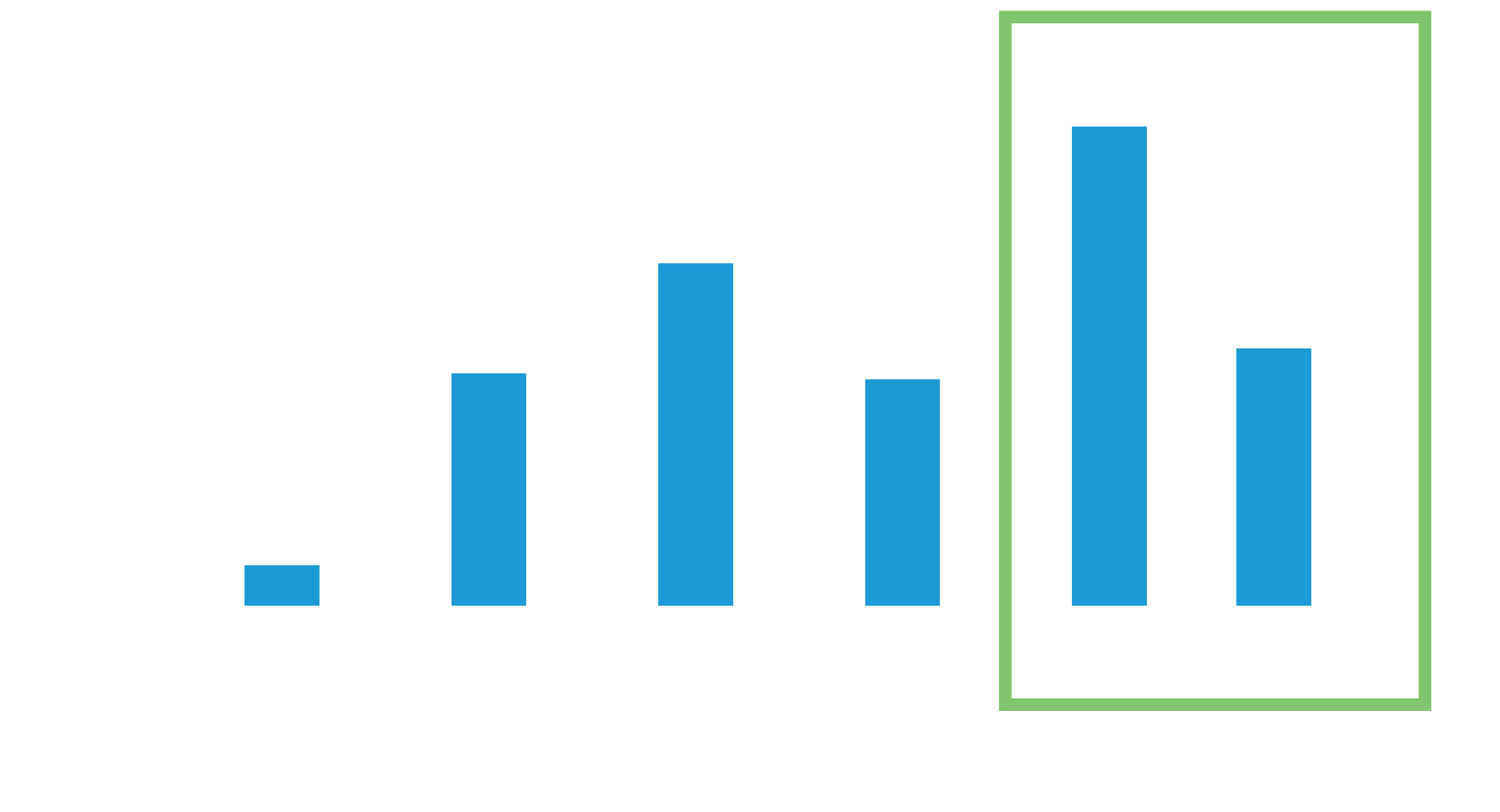
The average battery longevity among Leadless II phase 2 IDE patients at 1 year follow-up is estimated to be 17.6 years5
48% of the study patients have an estimated battery longevity of over 20 years.5
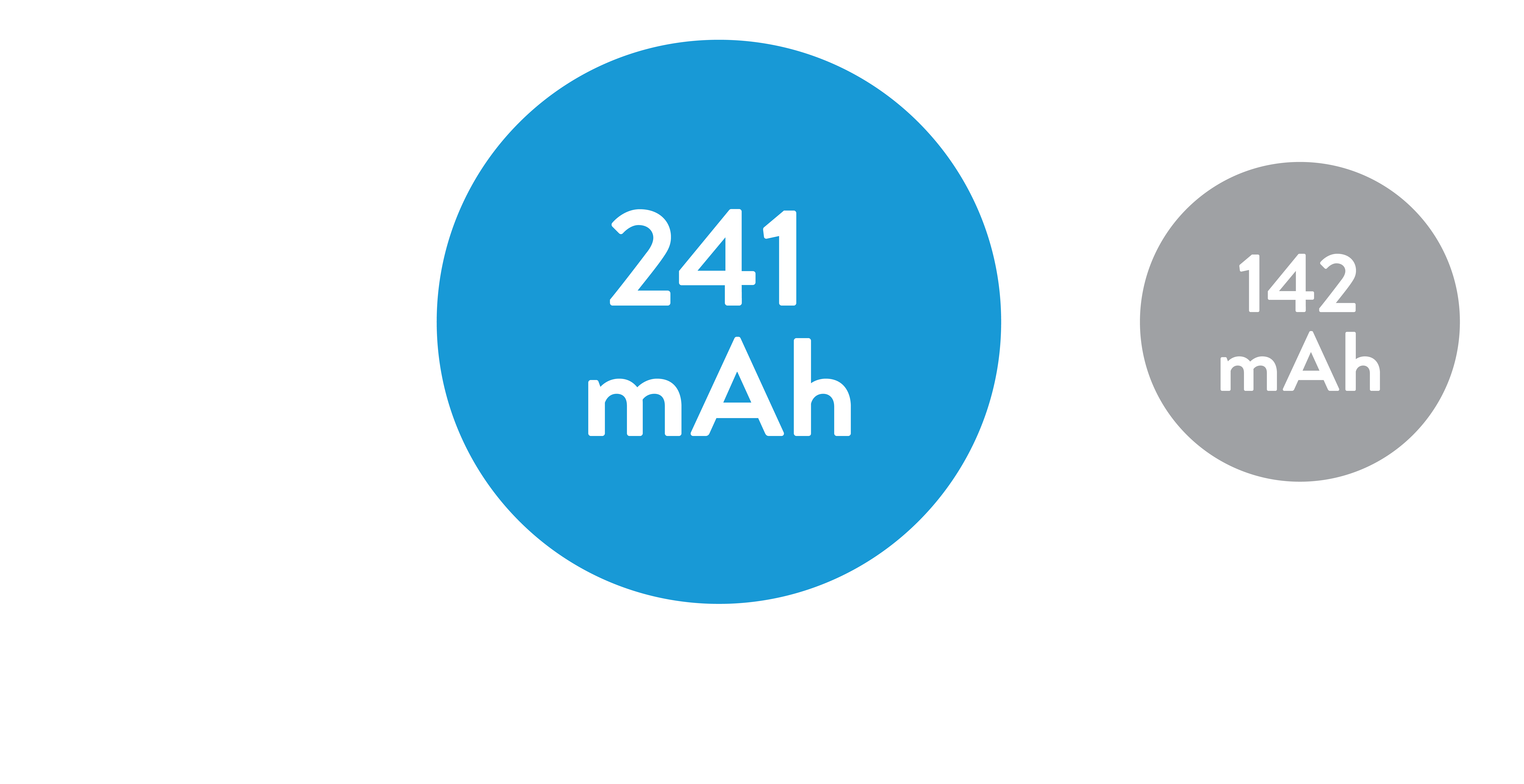
At least
1.7x the Battery Capacity
of current ventricular leadless pacemakers3,5*
Upgradeable System1
Patient therapy can be tailored by implanting a ventricular device alone, or both an atrial and ventricular device combined for dual chamber support. The option to upgrade over time allows you to meet your patient’s needs today and adapt to common disease progression later.
Treat rare intermittent heart block today.
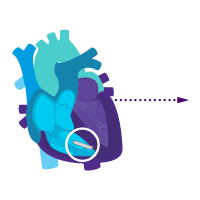
Add an atrial device for sick sinus syndrome later.
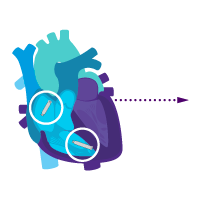
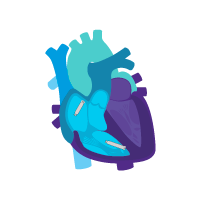
Enable i2i™ communication.

Activate dual chamber pacing therapy DDD(R) via i2i communication.
References
* Battery longevity estimates based on projections derived from published technical specifications and the ISO standard settings
**For additional information about specific MR Conditional, including warnings, precautions, adverse conditions to MRI scanning and potential adverse events, please refer to the MRI-Ready Leadless Systems Manual at medical.abbott/manuals or check our MRI-Ready resources at cardiovascular.abbott/mriready
- AVEIR Leadless Pacemakers and Delivery Catheter IFU. ARTEN600361109.
- Neuzil, Petr, et al. “Worldwide Chronic Retrieval Experience of Helix-Fixation Leadless Cardiac Pacemakers.” Journal of the American College of Cardiology, 2025.
- AVEIR™ VR Leadless Pacemaker and Delivery Catheter IFU. ARTEN600384624.
- Micra‡ VR2 MC2VR01 IFU.
- Reddy VY, Exner D, et al. 1-Year Outcomes of a Leadless Ventricular Pacemaker: The LEADLESS II (Phase 2) Trial. JACC : Clinical Electrophysiology 2023, DOI: 10.1016/j.jacep.2023.01.031
Additional References
- Sattar et al. Complications of leadless vs conventional (lead) artificial pacemakers - a retrospective review. Journal of community hospital internal medicine perspectives vol. 10,4 328-333. 2 Aug. 2020, doi:10.1080/20009666.2020.178690
- Reddy VY, Cantillon DJ, John IP. San Francisco, CA: 6 May 2016. A comparative study of acute and mid-term complications of leadless vs transvenous pacemakers. Late-Breaking Clinical Trials II. Presented at Heart Rhythm Society 2016; pp. 02–04. Abstract LBCT.
MAT-2406796 v3.0