Experience Advanced Detection Accuracy
Capture arrhythmia episodes correctly by significantly reducing false detections.
SharpSense™ technology harnesses the power of extra discriminators to confirm or reject detections - improving accuracy.1,2
Improve detection specificity with SharpSense technology
SharpSense technology is designed with more discriminators, improving specificity while maintaining a high relative sensitivity, to give you actionable information you can use to confidently diagnose bradycardia, pause and AF.1
SharpSense™ Technology has led to a 97% Reduction in False Detection of Events2*
Atrial Fibrillation
Actively reviews the previous 30 seconds for the presence of P-waves to discern an AF episode.
Relative Sensitivity: 97.2%1
Bradycardia
Dynamically evaluates multiple R-waves and P-waves to create customized thresholds to detect true bradycardia episodes.
Relative Sensitivity: 98.6%1
Pause
Analyzes P-wave and R-wave characteristics during the previous 6 seconds, based on customized secondary thresholds, to detect true pause episodes.
Relative Sensitivity: 98.1%1
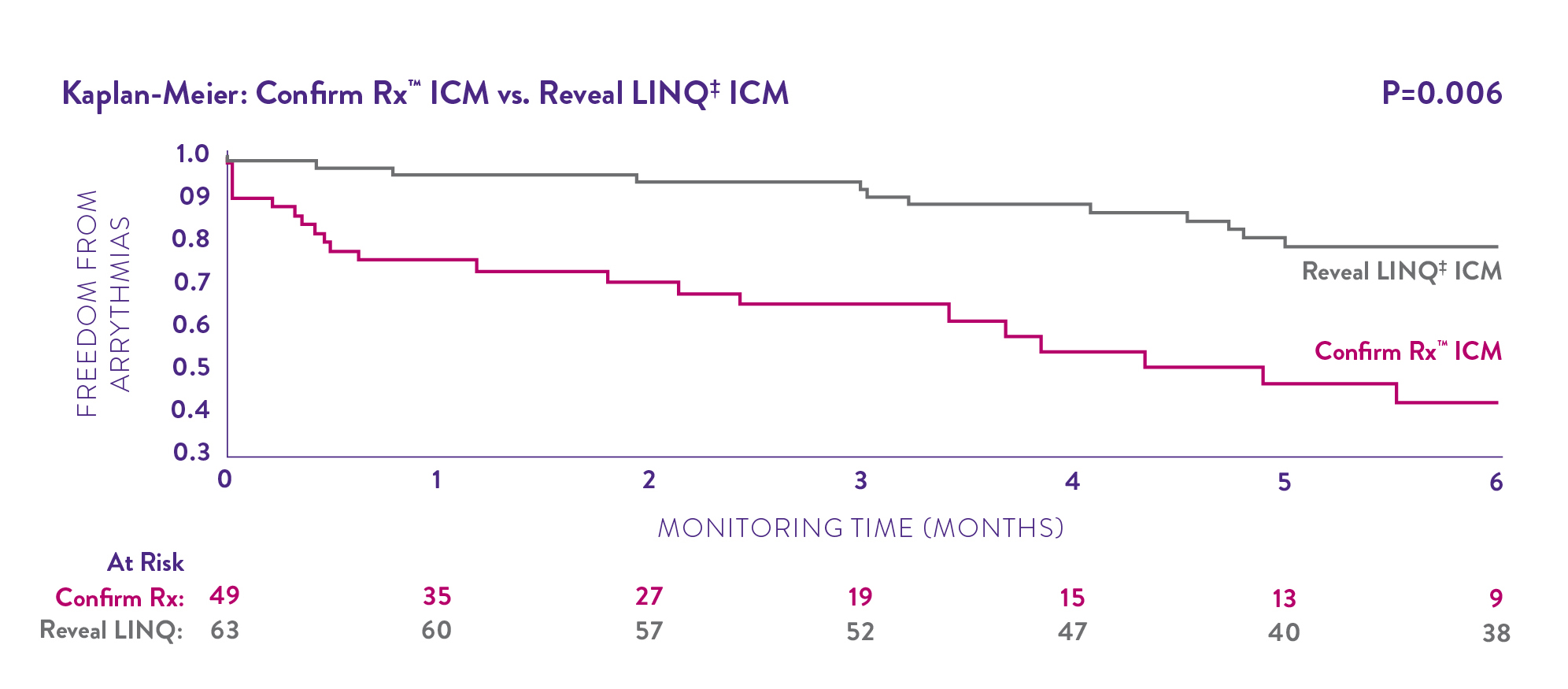
Kaplan-Meier analysis showed that the Confirm Rx ICM device detected significantly more true arrhythmic episodes in more patients - and did so sooner.3
Diagnose difficult-to-detect arrhythmias
The Confirm Rx ICM device continuously monitors patient rhythm for up to 2 years (730 days).6
84 Days
Median time to detect an AF episode in patients with Cryptogenic Stroke when using an implantable loop recorder.4†
134 Days
Median time for diagnosis in patients with Unexplained Syncope when using an implantable loop recorder.5
730 Days
Confirm Rx ICM offers up to 730 days of continuous heart monitoring — providing ample time to diagnose the most difficult-to-detect arrhythmias.6
This device is commercially available for use in select international markets.
*Determined by evaluation of real-world episodes in previously identified devices, using SharpSense Technology
† This information was not collected with the Confirm Rx ICM Device and is meant to provide information to the broader class of devices.
References
- Data on File. Abbott - Report 60076435; Design Validation Trace Matrix for Confirm Rx ICM System.
- Data on File. Abbott - Report 60098828; Evaluation of Clinic Impact of Confirm Rx ICM 1.2 Algorithm Enhancements.
- Ip J, Jaffe B, Castellani M, et al. Arrhythmia Detection in Implantable Cardiac Monitor Randomized Clinical Trial Comparing Reveal LINQ and Confirm Rx. PACE. Sept. 24, 2020.https://doi.org/10.111/pace.14076.
- Sanna T, Diener H-C, Passman RS, et al. Cryptogenic Stroke and Underlying Atrial Fibrillation. The New England Journal of Medicine. 2014;370(26):2478-2486. doi:10.1056/NEJMoa1313600.
- Solbiati M, Casazza G, Dipaola F, et al. The Diagnostic Yield of Implantable Loop Recorders in Unexplained Syncope: A Systematic Review and Meta-Analysis. International Journal of Cardiology. 2017;231:170-176. doi:10.1016/j.ijcard.2016.12.128.
- Abbott. Confirm Rx ICM User Manual.
MAT-2012211 v2.0
