The Abbott portfolio of surgical valves includes the Epic™ Platform of stented tissue valves consisting of the Epic™ Max and Epic™ Plus valves, and the Masters Series and Regent™ mechanical heart valves which are considered the gold standard and most trusted mechanical heart valves in the world. Learn more about the stented tissue valves and mechanical valves.
Tissue Heart Valves
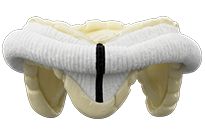
Epic Max
Epic™ Max Stented Tissue Valve
The Epic™ Max aortic valve with Linx™ anticalcification technology is designed to provide unparalleled hemodynamics* while maintaining the Epic Platform's ease of implant, proven durability, and features designed for future intervention.
*Compared to previous Epic Platform iterations.
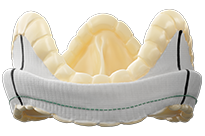
Epic Plus
Epic™ Plus Supra Stented Tissue Valve
The Epic™ Plus Supra aortic valve with Linx™ anticalcification technology is engineered to deliver excellent durability and performance, smooth & streamlined implantability, and features designed to facilitate valve-in-valve* in the aortic position.
*The safety and effectiveness of valve-in-valve procedures in an Epic™ Plus or Epic™ Plus Supra valve have not been established.
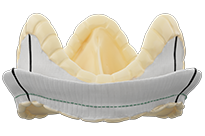
Epic™ Plus Mitral Stented Tissue Valve
The Epic™ Plus mitral valve with Linx™ anticalcification technology is engineered to deliver durability and performance, excellent implantability with the low profile, one cut holder, and TMVR valve-in-valve* compatibility.
*The safety and effectiveness of valve-in-valve procedures in an Epic™ Plus or Epic™ Plus Supra valve have not been established.
Mechanical Heart Valves
Regent™ Mechanical Heart Valve
The Regent™ aortic mechanical heart valve delivers exceptional hemodynamics and clinical performance with a proven long-term legacy of structural integrity and durability.

Masters Series Mechanical Heart Valve
The Masters Series aortic and mitral mechanical heart valves lead the way with a proven bileaflet design that results in low thrombogenicity and excellent patient outcomes. Unique design features have established Abbott mechanical heart valves as the gold standard for reliability and performance.

MAT-2011952 v3.0
EPIC™ PLUS/ EPIC™ PLUS SUPRA

Indications for Use
The Epic™ Plus valve is indicated for patients requiring replacement of a diseased, damaged, or malfunctioning native aortic and/or mitral heart valve. It may also be used as a replacement for a previously implanted aortic and/or mitral prosthetic heart valve.
The Epic™ Plus Supra valve is indicated for patients requiring replacement of a diseased, damaged, or malfunctioning native aortic heart valve. It may also be used as a replacement for a previously implanted aortic prosthetic heart valve.
Contraindications
None known.
Potential Adverse Events
Adverse events potentially associated with the use of bioprosthetic heart valves (in alphabetical order) include: angina; cardiac arrhythmias; endocarditis; heart failure; hemolysis; hemolytic anemia; hemorrhage, anticoagulant/antiplatelet-related; leak, transvalvular or paravalvular; myocardial infarction; nonstructural dysfunction (entrapment by pannus or suture, inappropriate sizing or positioning, or other); prosthesis regurgitation; stroke; structural deterioration (calcification, leaflet tear, or other); thromboembolism; valve thrombosis.
It is possible that these complications could lead to: reoperation; explantation; permanent disability; death.
EPICTM MAX STENTED PORCINE TISSUE VALVES

Indications for Use
The Epic™ Max valve is indicated for patients requiring replacement of a diseased, damaged, or malfunctioning native aortic heart valve. It may also be used as a replacement for a previously implanted aortic prosthetic heart valve.
Contraindications
None known.
Potential Adverse Events
Adverse events potentially associated with the use of bioprosthetic heart valves (in alphabetical order) include: angina; cardiac arrhythmias; endocarditis; heart failure; hemolysis; hemolytic anemia; hemorrhage, anticoagulant/antiplatelet-related; leak, transvalvular or paravalvular; myocardial infarction; nonstructural dysfunction (entrapment by pannus or suture, inappropriate sizing or positioning, or other); prosthesis regurgitation; stroke; structural deterioration (calcification, leaflet tear, or other); thromboembolism; valve thrombosis. It is possible that these complications could lead to: reoperation; explantation; permanent disability; death.
Regent™ Mechanical Heart Valve

Indications for Use
The SJM Regent™ Mechanical Heart Valve is intended for use as a replacement valve in patients with a diseased, damaged, or malfunctioning aortic valve. This device may also be used to replace a previously implanted aortic prosthetic valve.
Contraindications
The SJM Regent™ Mechanical Heart Valve is contraindicated for individuals unable to tolerate anticoagulation therapy.
Potential Adverse Events
Complications are associated with replacement mechanical heart valves include, but are not limited to, hemolysis, infections, thrombus, or thromboembolism, valve dehiscence, unacceptable hemodynamic performance, hemorrhagic complications secondary to anticoagulation therapy, prosthetic failure, heart failure or death. Any of these complications may require reoperation or explantation of the device.
Masters Series Mechanical Heart Valve

Indications for Use
The Masters Series Mechanical Heart Valve is intended for use as a replacement valve in patients with a diseased, damaged, or malfunctioning aortic or mitral heart valve. This device may also be used to replace a previously implanted mitral or aortic prosthetic valve.
Contraindications
The Masters Series Mechanical Heart Valve is contraindicated for individuals unable to tolerate anticoagulation therapy.
Potential Adverse Events
Complications associated with replacement mechanical heart valves include, but are not limited to, hemolysis, infections, thrombus, or thromboembolism, valve dehiscence, unacceptable hemodynamic performance, hemorrhagic complications secondary to anticoagulation therapy, prosthetic failure, failure or death. Any of these complications may require reoperation or explantation of the device.
