HeartMate II Studies
MOMENTUM 3: A Fully Magnetically Levitated Left Ventricular Assist Device — Final Report
Mehra MR, Uriel N, Yoshifumi N, et al.
HeartMate II was the LVAD used as a control in this pivotal trial. (Funded by Abbott; MOMENTUM 3 ClinicalTrials.gov number, NCT02224755.) The axialflow pump, which has the most implants and longest follow-up period for any LVAD in use today, performed well, with the following key data points at 2 years:
Survival – 76.7%
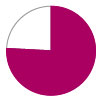
Incidence of stroke – 19.4%
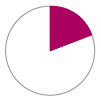
Incidence of GI bleeding – 30.9%
The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications
Teuteberg JJ, Cleveland JC, Cowger J, et al.
Real-world outcomes for HeartMate II LVAD were observed in the 2019 STS Intermacs annual report (Ann Thorac Surg 2020;109:649- 60), and were consistent with data observed in clinical trials at 2 years:
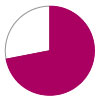
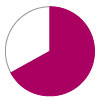
Survival –
72%
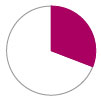
Freedom from stroke – 82%
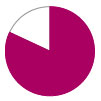
Freedom from GI bleed – 67%
Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: The ROADMAP Study 2-Year Results
Starling RC, Estep JD, Horstmanshof DA, et al.
Survival on original therapy with improvement in 6-min walk distance was superior with left ventricular assist device (LVAD) compared with optimal medical management (OMM) at 2 years. Reduction in key adverse events beyond 1 year was observed in the LVAD group. The ROADMAP trial provides risk-benefit information to guide patient- and physician-shared decision making for elective LVAD therapy as a treatment for heart failure. (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients [ROADMAP]; NCT01452802). (J Am Coll Cardiol HF 2017;)
Key data points:
- Better survival on original therapy with improvement in functional status for HeartMate II LVAD vs OMM
- More HeartMate II LVAD patients met primary endpoint (alive at 2 years and 6MWD improvement of at least 75 m)
- Functional class and QoL improvements
- Significantly more HeartMate II LVAD patients vs OMM were alive with improvements in functional capacity, quality of life, and depression vs OMM
MAT-2011123 v3.0
