
About
Proven Results for Long Term Support
The HeartMate II™ Left Ventricular Assist Device (LVAD) provides short or long-term circulatory support for intermediate-to-advanced heart failure patients. This small, quiet pump features a simple design — with only one moving part — and can provide blood flow equivalent to that of a healthy heart.
Over 26,000 patients implanted with the HeartMate II LVAD1
Unparalleled real-world experience
The HeartMate II LVAD is indicated for both destination therapy and bridge-to-transplantation in the United States. Additionally, HeartMate II LVAD is backed by more than 10 years of clinical experience.2
To date, more than 26,000 heart failure patients have received the HeartMate II LVAD.1 Many have passed the 5-year milestone on therapy, with some still on therapy after 15-plus years.1 These figures offer more than a decade of living proof that HeartMate II LVAD delivers predictable surgical and clinical performance for improved outcomes.3-8
Many patients have lived more than 15 years with HeartMate II LVAD1
Proven Long-Term Patient Outcomes
Research has shown the HeartMate II LVAD:
- Reverses and sustains reduction of heart failure symptoms8
- Increases patients’ survival rates and quality of life compared with medical management alone9
Predictable Performance for Improved Outcomes
Outstanding Two-Year Survival

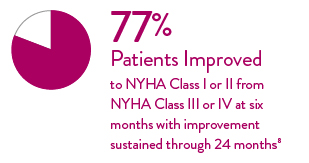
Significant Improvement in NYHA Class
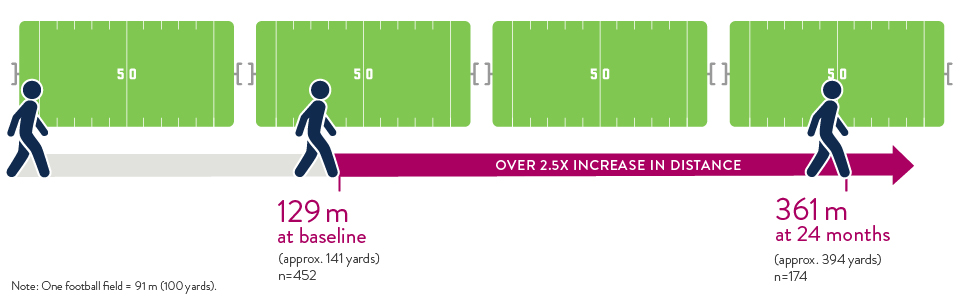
Significant Increase in 6-Minute Walk Distance
At baseline, patients who were tested completed the 6-minute walk test at an average of 129 meters. At 2 years after HeartMate II LVAD implant, patients completed the test at an average of 361 meters. That's two and a half times as far, for a 150% improvement in distance.8
Reduction in Thrombus with Adherence to PREVENT Guidelines
PREVENT Guidelines are a set of clinical and surgical recommendations that are aimed at maximizing flow through the LVAD, reducing risk of cannula malposition, and ensuring that the patients are adequately anti-coagulated while on LVAD support.

Patient-Centric Features
The HeartMate II LVAD is designed for patient comfort and convenience. With its wearable design and several carrying options, the HeartMate II LVAD supports your patients’ lifestyles.
Other features and benefits:
- Mean blood flow of up to 10 L/min—equal to that of a healthy heart
- Low-dose anticoagulation regimen
- Safe for air travel*
Safety by Design
The HeartMate II LVAD comes with a lightweight, pocket-sized controller that interfaces with the implanted pump.
Its features are designed for safety:
- Backup battery provides at least 15 minutes of full power in an emergency situation
- Prioritized visual alarms provide clear, actionable instructions
- On-screen instructions eliminate the need for guesswork
- Driveline diagnostic checks verify that driveline wires are intact and functional
*Please reference the HeartMate II LVAS Instructions for Use for additional information.
References
- Based on clinical trial and device tracking data as of May 31, 2025.
- Abbott Data on file as of July 20, 2020
- Jorde UP, Kushwaha SS, Tatooles AJ, et al. Results of the destination therapy post-food and drug administration approval study with a continuous flow left ventricular assist device: a prospective study using the INTERMACS registry (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol. 2014;63:1751-1757.
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495-1504.
- Estep JD, Starling RC, Horstmanshof DA, et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: results from the ROADMAP study. J Am Coll Cardiol. 2015;66:1747-61.
- Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med. 2017;376:451-60.
- Grady KL, Naftel D, Stevenson L, et al. Overall quality of life improves to similar levels after mechanical circulatory support regardless of severity of heart failure before implantation. J Heart Lung Transplant. 2014:33(4):412-421.
- Mehra M, Uriel N, Naka Y, et al. A Fully Magnetically Levitated Ventricular Assist Device-Final Report. N Engl J Med. 2019;380:1618-1627.
- Starling RC, Estep JD, Horstmanshof DA, et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: The ROADMAP Study 2-Year Results. J Am Coll Cardiol HF. 2017;5:518-527.
- Maltais S, Kilic A, Natha S, et al. PREVENtion of HeartMate II Pump Thrombosis Through Clinical Management: The PREVENT multi-center study. J Heart Lung Transplant. 2017;36:1-12.
MAT-2010996 v3.0
