True Electrograms* With Ensite™ Omnipolar Technology (OT)
Accelerate diagnosis & treatment decisions through accurate mapping with EnSite Omnipolar Technology and the Advisor™ HD Grid Mapping Catheter, Sensor Enabled™¹.
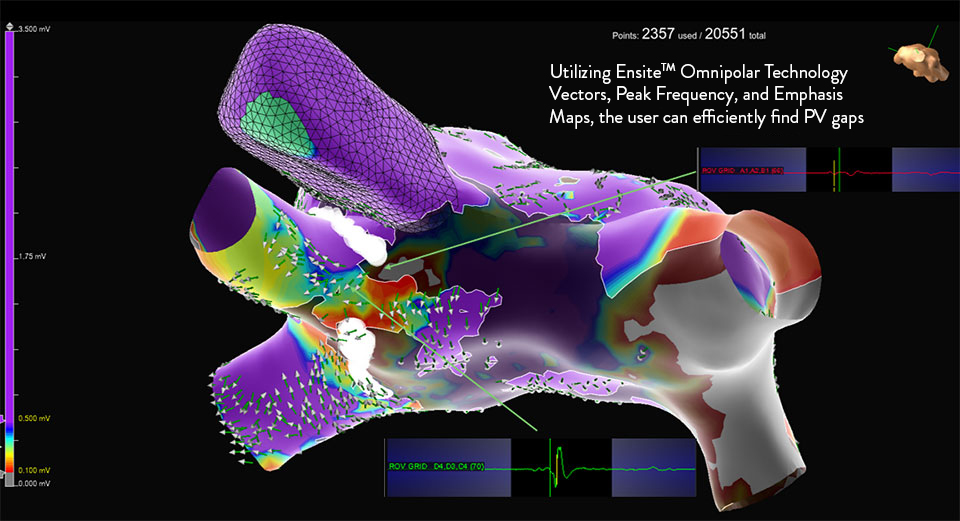
What is Omnipolar Technology?
Omnipolar technology accelerates diagnosis and treatment decisions by providing a new way to calculate bipolar electrograms that are independent from catheter wavefront orientation. EnSite OT maximizes data collection and increases map resolution by tripling the number of points collected per save as points are effectively placed every 2 mm¹**.
*OT captures true signals independent of catheter orientation relative to the wavefront.
**As compared to the use of traditional bipole mapping.
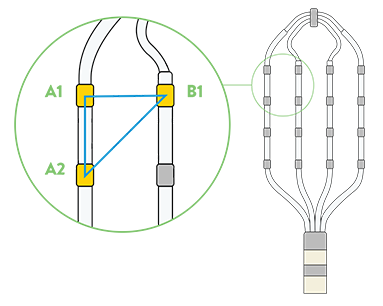
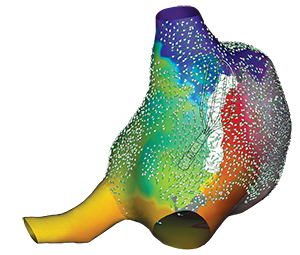
Identify True, Local
annotation-independent tissue characteristics¹
Gain Instant New Perspective of Wavefront Direction
with activation direction arrows displayed beat by beat
See Every Signal in 360 Degrees -
capturing signals that no other mapping technology can see**
Enhance Map Accuracy
with 3x increase in point density per acquisition***1
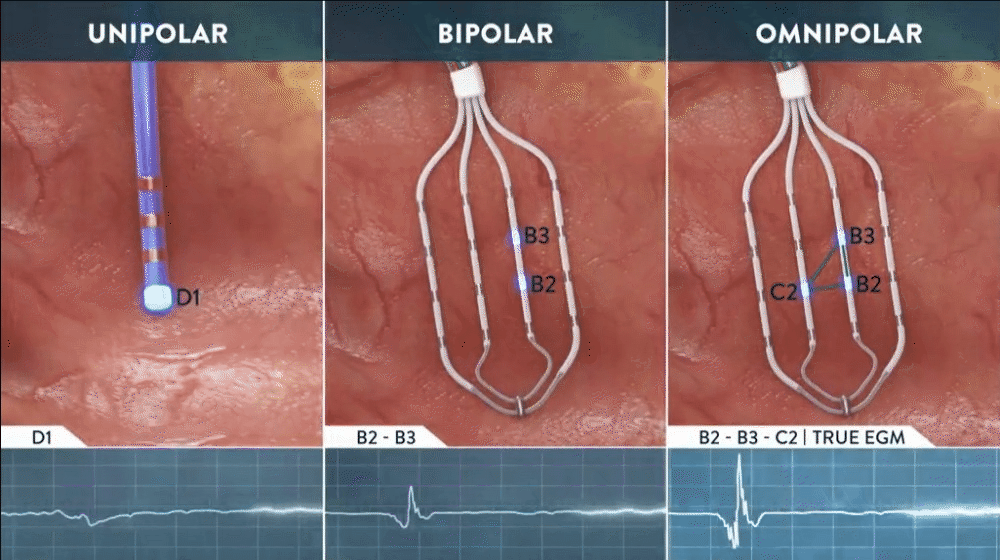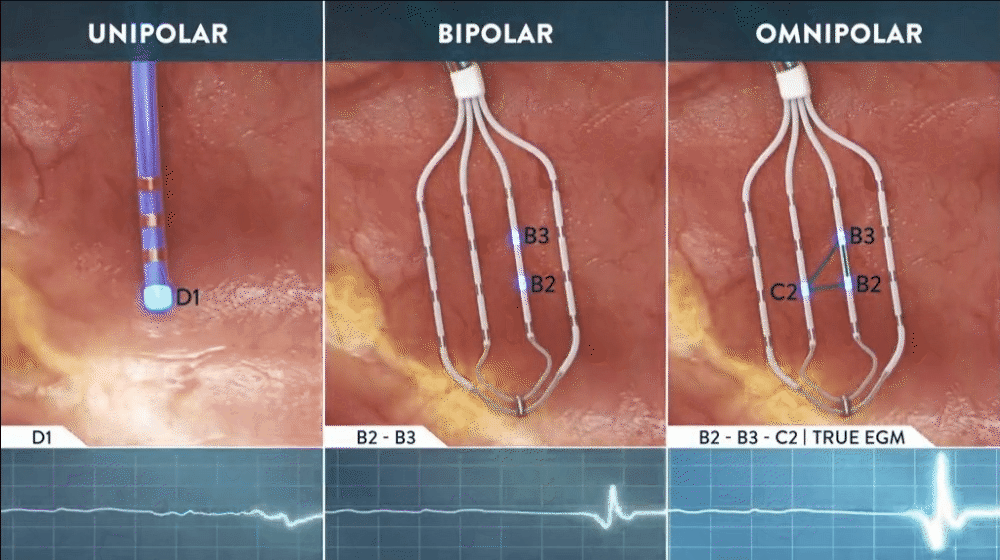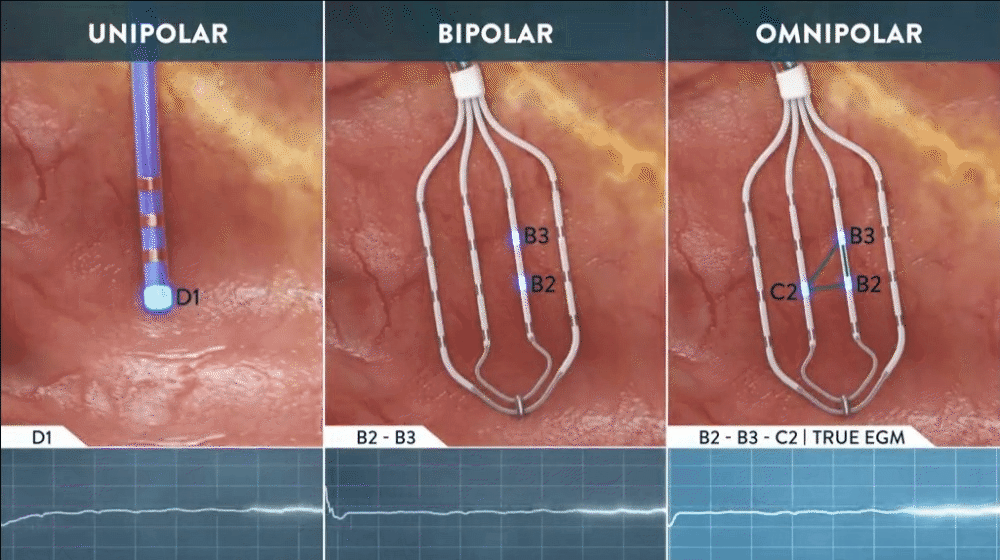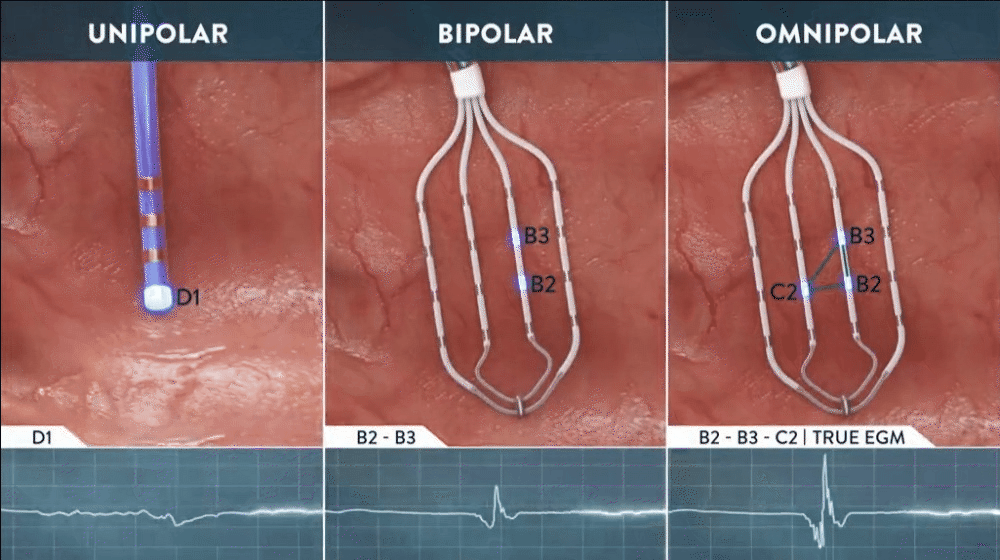
**Every signal can be defined as any signal seen on the RAI window recorded by the HD Grid mapping catheter when the map polarity is set to omnipolar.
***As compared to the use of traditional bipole mapping.
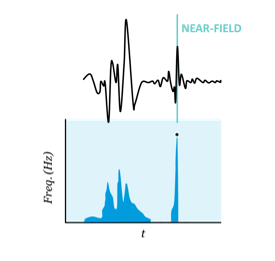
What is EnSite™ OT Near Field?
Further building on the EnSite™ Omnipolar Technology revolution, EnSite OT Near Field is a new mapping technology that automates point annotation by isolating true localized signals.
- Simple, objective and automated assessment of near field signals
- Gain unique insights on a substrate and tissue contact with new peak frequency data points
- Streamline map interpretation by emphasizing key information in related data sets
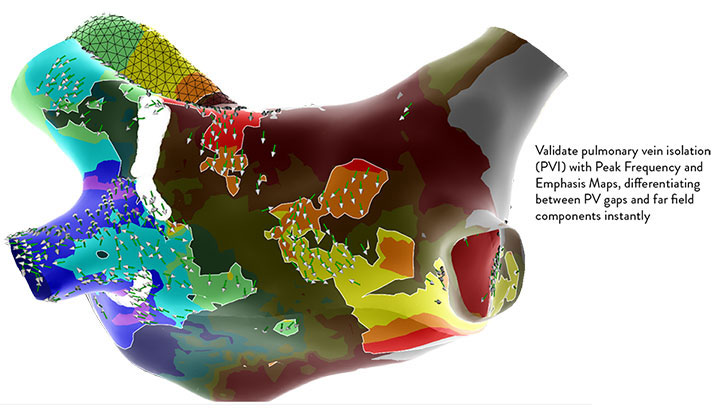
True Localized Signals, More Confident Insights
Annotation
Nearfield EGM Aquisition
Simple Objective, and Automated assessment of near-field signals
ENSITE™ OT NEAR FIELD algorithm uses the Signal Frequency (Hz) to analyze the EGMs and assess Near Field vs. Far Field
- EnSite™ OT Near Field continuously analyses signal frequency to determine peak frequency which correlates to the sharpest signal component by identifying the Near-Field.
- Detects EGM sharpness, independent of amplitude
- Other detection algorithms may favor annotation of signals with higher amplitudes +dV/dt, -dV/dt, Abs dV/dt, Min Voltage, Max Voltage
- Available for omnipolar and bipolar signals
Interpretation
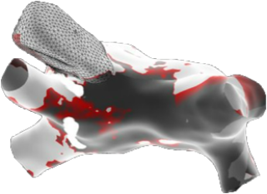
Peak Frequency Maps
Gain Unique Insights on Substrate and Tissue Contact with New Peak Frquency Data Points
Peak Frequency Map is a new map type that displays the high-frequency activity based upon the peak frequency of each electrogram.
- Allows the user to discriminate areas of near field vs. far-field activity..
- Near field (high frequency) areas are displayed in white, while far field (low frequency) areas are displayed in a darker gray color.
Interpretation
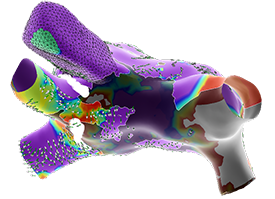
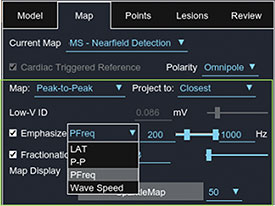
Emphasis Maps
STREAMLINE MAP INTERPRETATION BY EMPHASIZING KEY INFORMATION IN RELATED DATA SETS
- Focus on the target of interest
- Define the metrics for your success
- Increase specificity for ablation strategy
1. Deno, D. C., Bhaskaran, A., Morgan, D. J., Goksu, F., Batman, K., Olson, G. K., Nanthakumar, K. (2020). High-resolution, live, directional mapping. Heart Rhythm, 17(9), 1621-1628. doi:10.1016/j.hrthm.2020.04.039
MAT-2601169 v1.0
Important Safety Information
Rx Only. Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events, and directions for use.
United States: Required Safety Information
Indications: The EnSite™ X EP System is a suggested diagnostic tool in patients for whom electrophysiology studies have been indicated. The EnSite™ X EP System provides information about the electrical activity of the heart and displays catheter location during conventional electrophysiological (EP) procedures. Warnings: For patient safety, any connections that directly connect the patient to the EnSite™ X EP System must be routed through the appropriate modules: EnSite™ X EP System SurfaceLink Module, EnSite™ X EP System 20 pin Catheter Input Module, EnSite™ X EP System 80-pin Catheter Input Module and Direct Connect Ports on the EnSite™ X EP System Amplifier. When using the EnSite™ X EP System, full protection against the effects of cardiac defibrillator discharge and other leakage currents is dependent upon the use of appropriate cables. Refer to the ablation catheter IFU for a listing of adverse events related to the use of this device in conjunction with ablation, as a part of the diagnosis and treatment of cardiac arrhythmias. Non-SE catheters cannot collect location data and should not be used for navigation in VoXel Mode because they do not have a magnetic sensor. However, they can be visualized and display intracardiac signals. Only connect items that have been specified as part of the EnSite X EP System or compatible with the EnSite X EP System to the multiple socket-outlets. The EnSite™ X EP System model display should be used in conjunction with conventional EP techniques to confirm catheter location. The AutoMark feature does not indicate lesion effectiveness. AutoMarks are placed based on user-defined parameters for catheter stability and RF metrics only. PFA AutoMarks are placed based on electrode location and user-defined PFA metrics only. Sudden impedance changes of the body or catheter electrodes caused by the connection of other devices (e.g., stimulator, defibrillator, and other devices) may create a location shift. The 2D and 3D LivePoint Displays should not be used as the primary / sole display of tissue proximity during an Electrophysiology study. Refer to the Current PFA Generator IFU for warnings related to the Volt ™ LivePoint Display. Precautions: For patient safety, any connections that directly connect the patient to the EnSite™ X EP System must be routed through the appropriate modules: EnSite™ X EP System SurfaceLink Module, EnSite™ X EP System 20 pin Catheter Input Module, EnSite™ X EP System 80-pin Catheter Input Module and Direct Connect Ports on the EnSite™ X EP System Amplifier. When using the EnSite™ X EP System, full protection against the effects of cardiac defibrillator discharge and other leakage currents is dependent upon the use of appropriate cables. Refer to the ablation catheter IFU for a listing of adverse events related to the use of this device in conjunction with ablation, as a part of the diagnosis and treatment of cardiac arrhythmias. Non-SE catheters cannot collect location data and should not be used for navigation in VoXel Mode because they do not have a magnetic sensor. However, they can be visualized and display intracardiac signals. Only connect items that have been specified as part of the EnSite X EP System or compatible with the EnSite X EP System to the multiple socket-outlets. The EnSite™ X EP System model display should be used in conjunction with conventional EP techniques to confirm catheter location. The AutoMark feature does not indicate lesion effectiveness. AutoMarks are placed based on user-defined parameters for catheter stability and RF metrics only. PFA AutoMarks are placed based on electrode location and user-defined PFA metrics only. Sudden impedance changes of the body or catheter electrodes caused by the connection of other devices (e.g., stimulator, defibrillator, and other devices) may create a location shift. The 2D and 3D LivePoint Displays should not be used as the primary / sole display of tissue proximity during an Electrophysiology study. Refer to the Current PFA Generator IFU for warnings related to the Volt ™ LivePoint Display.
Indications for Use: The Advisor™ HD Grid X Mapping Catheter, Sensor Enabled™, is indicated for multiple electrode electrophysiological mapping of cardiac structures in the heart, i.e., recording or stimulation only. This catheter is intended to obtain electrograms in the atrial and ventricular regions of the heart. Contraindications: The catheter is contraindicated for patients with prosthetic valves, and patients with left atrial thrombus or myxoma, or interatrial baffle or patch via transseptal approach. This device should not be used with patients with active systemic infections. Patients unable to receive heparin or an acceptable alternative to achieve adequate anticoagulation. Warnings: Persons with a known history of allergies to any of the materials listed below may suffer an allergic reaction to this device. Before use, counsel the patient on the materials contained in the device and discuss a thorough history of allergies. This device contains: Acrylonitrilebutadienestyrene (ABS Cycolac) – Loctite Adhesive – Pellethane – Platinum Iridium alloy – Polyimide – Polyether block amide (PEBAX) – Polyethylene (High Density Polyethylene, HDPE) – Titanium. Cardiac catheterization procedures present the potential for significant xray exposure, which can result in acute radiation injury as well as increased risk for somatic and genetic effects, to both patients and laboratory staff due to the xray beam intensity and duration of the fluoroscopic imaging. Careful consideration must therefore be given for the use of this catheter in pregnant women. The safety and effectiveness of the device has not been established in pregnant women or prepubescent children. Careful consideration must therefore be given for the use of the device in pregnant women or prepubescent children. Catheter entrapment within the heart or blood vessels is a possible complication of electrophysiology procedures. Vascular perforation or dissection is an inherent risk of any electrode placement. Careful catheter manipulation must be performed in order to avoid device component damage, thromboembolism, cerebrovascular accident, cardiac damage, perforation, pericardial effusion, or tamponade. Risks associated with electrical stimulation may include, but are not limited to, the induction of arrhythmias, such as atrial fibrillation (AF), ventricular tachycardia (VT) requiring cardioversion, and ventricular fibrillation (VF). Do not use force to advance or withdraw catheter when resistance is encountered. Do not immerse the proximal handle or cable connector in fluids; electrical performance could be affected. Precautions: Maintain an activated clotting time (ACT) of greater than 300 seconds at all times during use of the catheter. This includes when the catheter is used in the right side of the heart. To prevent entanglement with concomitantly used catheters, use care when using the catheter in the proximity of the other catheters. Maintain constant irrigation to prevent coagulation on the distal paddle. Inspect irrigation tubing for obstructions, such as kinks and air bubbles. If irrigation is interrupted, remove the catheter from the patient and inspect the catheter. Ensure that the irrigation ports are patent and flush the catheter prior to reinsertion. Use the straightener during the insertion process to avoid damage to the hemostasis valve. Always straighten the catheter before insertion or withdrawal. Catheter advancement must be performed under fluoroscopic guidance to minimize the risk of cardiac damage, perforation, or tamponade. Compatible navigation and real time visualization systems may also be considered. Do not use if the catheter appears damaged, kinked, or if there is difficulty in deflecting the distal section to achieve the desired curve. Catheter materials are not compatible with magnetic resonance imaging (MRI). One or more components of this device may contain the following substance defined as CMR 1B in a concentration above 0.1% weight by weight: Nmethyl2pyrrolidone (NMP): Chemical Abstracts Service (CAS) No. 872504; EC No. 2128281. Based on a quantitative toxicological assessment it has been determined that NMP released from this device is unlikely to cause adverse reproductive effects. Potential Adverse Events: Complications related to the use of the device include, but are not limited to, the following: New or worsening of existing arrhythmia including Atrial fibrillation, Ventricular tachycardia requiring cardioversion, Supraventricular tachycardia (SVT) and Ventricular fibrillation Cardiac perforation including Pericardial effusion or Cardiac tamponade Bleeding including access site Hemorrhage / bleeding, Ecchymosis and Hematoma Vascular access complications or peripheral vascular injury including Femoral artery dissection, Dissection, Arteriovenous fistula and Pseudoaneurysm formation Pulmonary vein stenosis, Heart failure, Volume overload, Hypotension, Embolism, Cerebrovascular accident (CVA)/stroke, Infection, Pneumonia, Pulmonary edema, Immunological reaction, Pain and Pericarditis.