Versatile Solutions
The CPS locator 3D Plus catheters offer extended French sizes on the proven CPS Locator 3D platform for use with larger leads.
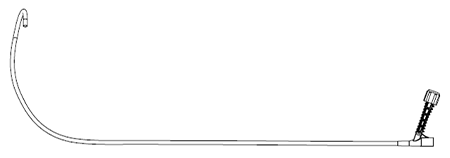
Large (DS4D300 -42/45)
The Large curve is designed to target the posterior/mid septum.
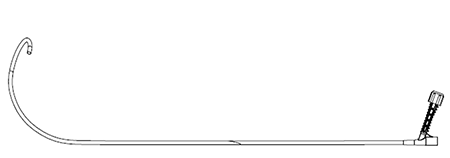
Extra Large (DS4D400 -42/45)
The Extra Large curve is designed to target the low septum.
Features of CPS Locator™ 3D Plus Catheters
- The CPS Locator™ 3D Plus Delivery Catheter is a single-use percutaneous catheter intended to introduce various types of catheters and pacing or defibrillator leads.
- Each CPS Locator™ 3D Plus Delivery Catheter offers a unique distal curve to accommodate various anatomies.
- The catheter is equipped with a hemostatic valve, and the distal tip is radiopaque to facilitate imaging under fluoroscopy.
- Slitter for CPS Locator™ 3D Plus Delivery Catheter is designed to ensure alignment and protect the lead during catheter slitting.
- Accommodates an 8F lead with a minimum length of 58 cm for the 42 cm catheters and 65 cm for the 45 cm catheters.
- The catheter has an inner diameter of 8.4F, an outer diameter of 10.2F and the dilator is compatible with a 0.035” guidewire.
Ordering Information
Model Number | Model Name | Overall Length (Cm) | Working Length (Cm) | Inner Diameter | Outer Diameter | Minimum Lead Length |
|---|---|---|---|---|---|---|
DS4D300-42 | CPS Locator™ 3D Plus, Large | 45 cm | 42 cm | 8.4F (2.80 mm) | 10.2F (3.40 mm) | 58 cm |
DS4D400-42 | CPS Locator™ 3D Plus, Extra Large | 45 cm | 42 cm | 8.4F (2.80 mm) | 10.2F (3.40 mm) | 58 cm |
DS4D300-45 | CPS Locator™ 3D Plus, Large, Extra Long | 48 cm | 45 cm | 8.4F (2.80 mm) | 10.2F (3.40 mm) | 65 cm |
DS4D400-45 | CPS Locator™ 3D Plus, Extra Large, Extra Long | 48 cm | 45 cm | 8.4F (2.80 mm) | 10.2F (3.40 mm) | 65 cm |
Accessories (available separately)
Accessory | Model Name | Model Number |
|---|---|---|
Slitter | CPS 3D Plus Slitter | DS4A001 |
Helix Locking Tool | DF4 Helix Locking Tool | A002HELX |
Stay Informed
Sign up to hear about our technology, education opportunities, and more.
Read the Latest Blog Article
Stay up to date with recent news, product highlights, and case studies.
Important Safety Information:
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
Indications for Use:
The Delivery Catheter is indicated for the introduction of various types of catheters and pacing or defibrillator leads.
Contraindications:
- Obstructed or inadequate vasculature for venous access
- Review contraindications associated with ancillary devices used with the Delivery Catheter
Complications: Possible complications associated with the Delivery Catheter include: Vessel trauma, Endocardial trauma, Blood loss, Lead Dislodgement, Embolization (material, air), Allergic reaction, Infection, Device incompatibility. Possible complications associated with the use of ancillary devices with the Delivery Catheter include: Air embolism, Bleeding, Bradycardia, Cardiac perforation, Cardiac tamponade, Chronic nerve damage, Death, Extracardiac stimulation (muscle/nerve stimulation), Hematoma, Heart block, Hemothorax, Incisional pain, Infection including endocarditis, Lead dislodgement, Myocardial infarction, Myocardial trauma, Pericarditis, Pneumothorax, Stroke, Tachyarrhythmias, Thrombosis/thromboemboli, Valve damage, Venous occlusion, Venous trauma (e.g., perforation, dissection, erosion).
Refer to the User’s Manual for detailed indications, contraindications, warnings, precautions, and potential adverse events.
Manufactured by:
CenterPoint Systems LLC
3338 Parkway Blvd West Valley City, UT 84119, U.S.A.
Tel: +1-877-848-0828
Info@centerpoint-systems.com
Distributed by:
Abbott Medical
15900 Valley View Court Sylmar, CA 91342 USA
+1-818-362-6822
MAT-2513907 v1.0