Clinical Evidence
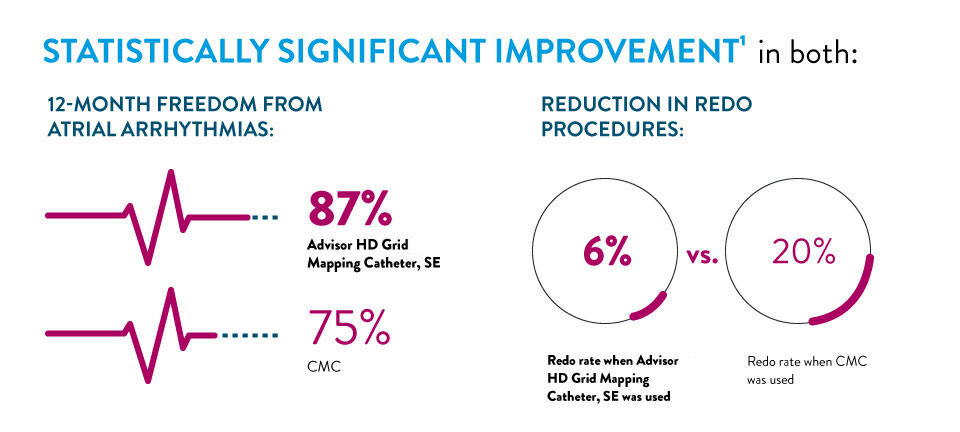
LONG-TERM DATA IN AF PROCEDURES SHOWS IMPROVED OUTCOMES WHEN USING ADVISOR™ HD GRID MAPPING CATHETER, SENSOR ENABLED™1
A new manuscript published by Dr. John Day, et. al comparing long-term outcomes of AF procedures completed with a circular mapping catheter (CMC) to those completed with the Advisor HD Grid Mapping Catheter, SE found that the use of the Advisor HD Grid Mapping Catheter, SE resulted in a:
WHEN YOU CHECK FOR GAPS IN PULMONARY VEIN ISOLATION, ARE YOU SEEING THEM ALL?
Acute data collection that includes both direct and indirect comparisons of the Advisor HD Grid Mapping Catheter, Sensor Enabled in standard pulmonary vein isolation (PVI) confirmation workflows suggests that the device can identify gaps that may be missed by other technologies.
Now Includes Expanded Data Sets
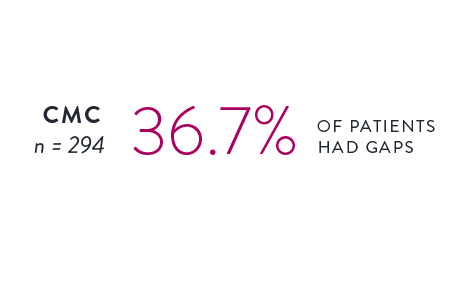
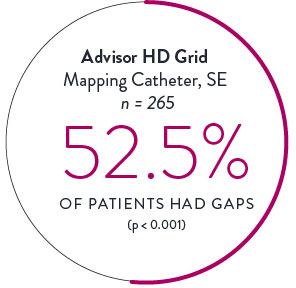
Circular Mapping Cathethers2
The incidence and location of gaps following PVI were tracked utilizing either a circular mapping catheter (CMC) or the Advisor HD Grid Mapping Catheter, SE.
Isolation was tracked across 559 cases
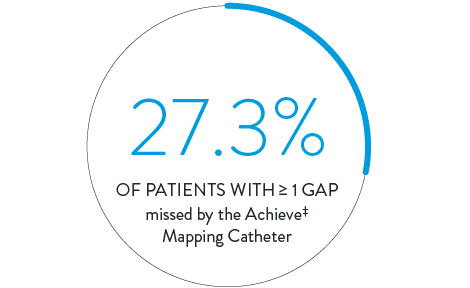
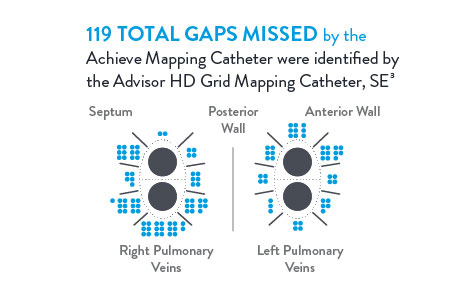
Cryoablation3
In a direct comparison, 150 patients received cryoballoon ablation with isolation confirmed by the Achieve‡ Mapping Catheter. Isolation was then checked again with the Advisor HD Grid Mapping Catheter, SE, revealing:
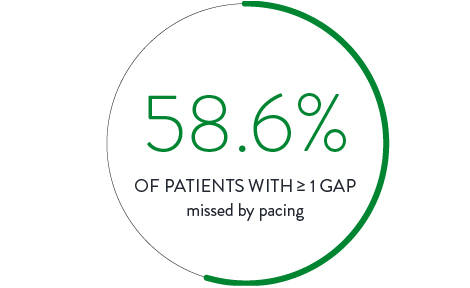
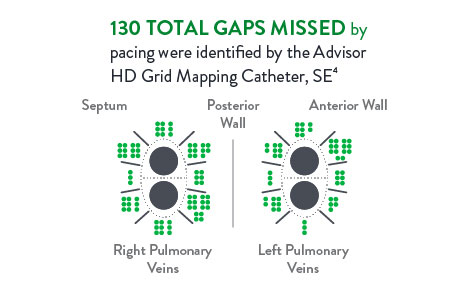
Pacing Ablation Line4
In a direct comparison, 111 patients received ablation with isolation confirmed by pacing the ablation line. Isolation was then checked again with the Advisor HD Grid Mapping Catheter, SE, revealing:
References
- Day, J. D., Crandall, B., Cutler, M., Osborn, J., Miller, J., Mallender, C., & Lakkireddy, D. (2020). High Power Ultra Short Duration Ablation with HD Grid Improves Freedom from Atrial Fibrillation and Redo Procedures Compared to Circular Mapping Catheter. Journal of Atrial Fibrillation, 13(2).
- Porterfield, C., Rillo, M., Wystrach, A., Rossi, P., Zedda, A. M., Mine, T., Mantovan, R., Favilla, A., & Nilsson, K. (2021). Assessment and incidence of PV gaps as determined by HD Grid and circular mapping catheters. EP Europace, 23(Supplement_3). https://doi.org/10.1093/europace/euab116.194
- Gaitonde, R. S., Martel, J. A., Kobori, A., Koide, N. S., Altemose, G. T., Eldadah, Z., Baher, A., Dell”orfano, J., Gora, P., & Mathew, S. (2021). Incidence of residual gaps identified by a high-density grid-style catheter post-cryoballoon ablation for atrial fibrillation. EP Europace, 23(Supplement_3). https://doi.org/10.1093/europace/euab116.197
- Giuggia, M., Volpicelli, M., Mantica, M., Notarangelo, M. F., Sundaram, S., Gora, P., & Bottoni, N. (2021). Incidence and location of residual gaps identified by a high-density grid-style catheter after PVI is confirmed by pacing the ablation lines. EP Europace, 23(Supplement_3). https://doi.org/10.1093/europace/euab116.190
MAT-2007563 v7.0
