What is a Pacemaker?
It's a heart rate manager.
A pacemaker is a small, battery-powered device that “listens” to your heart. If it’s beating properly, the pacemaker does nothing. But when your heart is beating too slowly, the pacemaker sends tiny electrical signals to return your heart rate to your normal range.
It can evaluate your heart.
It also records and stores information about your heart rate and any potential arrhythmias for your doctor to plan for the best treatments and therapies.
What are the Types of Pacemakers?
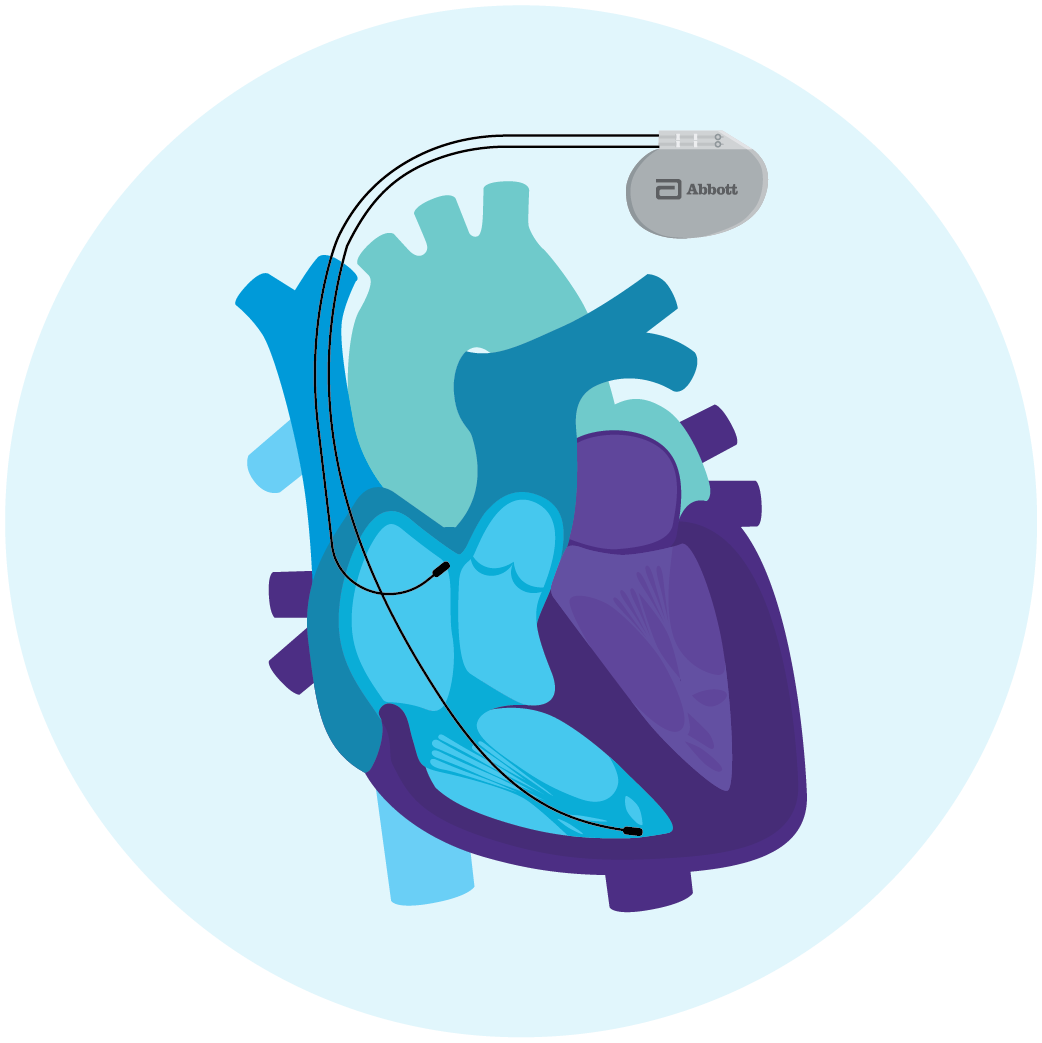
Traditional Pacemaker with Leads
A traditional pacemaker is implanted under the skin in the chest area and connected to leads that travel down blood vessels to the heart and attach to the heart tissue. Leads are flexible, insulated wires that run between the pacemaker and the tissue of the heart. If the pacemaker senses that your heart has slowed down or missed a beat, the leads transmit electrical impulses to the heart to restore it to its normal rate.

Leadless Pacemaker
A leadless pacemaker functions in a similar way to control your heart rate, but achieves this in a different way. Instead of using leads, the pacemaker can sense and pace your heart as an all-in-one small device implanted in your heart through a blood vessel in your leg. Unlike a traditional pacemaker, it does not have any leads or wires, and does not require a chest incision, so no scars on the chest or arm movement restrictions.

Leadless pacemakers are 10 times smaller than a traditional pacemaker and do not require leads.1, 2
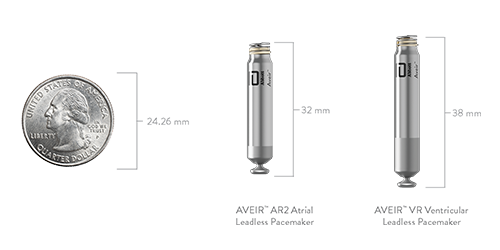
AVEIR™ Leadless Pacemaker Indications:
AVEIR AR2 Atrial
Leadless Pacemaker
- Sinus node dysfunction and normal AV and intraventricular conduction systems
AVEIR VR Ventricular
Leadless Pacemaker
- Significant bradycardia and normal sinus rhythm with only rare episodes of AV block or sinus arrest
- Chronic atrial fibrillation
AVEIR DR Dual Chamber
Leadless Pacemaker System
- Sick sinus syndrome
- Chronic, symptomatic second- and third-degree AV block
- Symptomatic bilateral bundle-branch block when tachyarrhythmia and other causes have been ruled out
{hospital}
{distance} miles
Results
* This finder is compiled by Abbott as a reference tool for locating or identifying certain physicians. The physicians included in this database are those who have undergone the AVEIR™ VR Physician Implant Training Program or the AVEIR™ DR Physician Implant Training Program by Abbott and then requested inclusion in the finder. This finder may not include all physicians that have undergone such training, and/or use or have used AVEIR. Similarly, this finder does not include all physicians who practice in the field of cardiology. No physician has been paid or received a fee to be included in the finder.
Abbott does not verify or monitor the license, credentials, accreditations, or qualifications of any physician listed, and is not responsible for the medical advice of the physicians included in this finder. Abbott is not responsible and shall not be liable to you or others for any decision made or action taken by you in reliance on this information obtained from this finder.
Inclusion of a physician in this finder does not mean that Abbott endorses or recommends such physician. Similarly, the fact that a physician is not included in the finder does not mean that the physician is not qualified.
The results are provided by proximity when searching by zip code, city, or state. Results are only available for physicians in the United States.
Consult your physician about your condition and treatment options. Third-party payor criteria may also apply.
Physicians meeting the inclusion criteria who are not currently listed on the finder and who wish to be included, may contact aveirlookup@abbott.com.
Hear Stories from Patients with AVEIR Leadless Pacemakers
Mikey
As a lifelong, competitive surfer, Mikey's ability to surf was put on hold after multiple traditional pacemaker surgeries until he discovered the AVEIR DR Dual Chamber Leadless Pacemaker System.
Bryce
From Saving Lives to Living Fully Again: First-Responder Bryce's Journey to AVEIR DR Dual Chamber Leadless Pacemaker System
Dennis
Being active and independent is important to Dennis, which is why his doctor recommended an AVEIR™ DR Dual Chamber Leadless Pacemaker System.
Chelsey
Young, vibrant, active – and living her best life, Chelsey needed a pacemaker, and the AVEIR™ VR Leadless Pacemaker was the ideal solution for her active lifestyle.

Mikey
As a lifelong, competitive surfer, Mikey's ability to surf was put on hold after multiple traditional pacemaker surgeries until he discovered the AVEIR DR Dual Chamber Leadless Pacemaker System.

Bryce
From Saving Lives to Living Fully Again: First-Responder Bryce's Journey to AVEIR DR Dual Chamber Leadless Pacemaker System

Dennis
Being active and independent is important to Dennis, which is why his doctor recommended an AVEIR™ DR Dual Chamber Leadless Pacemaker System.

Chelsey
Young, vibrant, active – and living her best life, Chelsey needed a pacemaker, and the AVEIR™ VR Leadless Pacemaker was the ideal solution for her active lifestyle.



What to Expect with Your Pacemaker
Find answers to your questions about traditional pacemakers, including the implantation procedure, safety and use, and daily living with your device.

AVEIR Leadless Pacemakers
Learn about the first-of-its-kind, dual chamber leadless pacing system and Abbott’s single chamber atrial and ventricular leadless pacemakers. These leadless pacemakers feature all the clinical benefits of traditional pacemakers without lead and pocket-related complications or arm movement restrictions.

Frequently Asked Questions
Pacing is the process in which regulating or changing the timing or intensity of your heartbeat, or cardiac contractions, are made. Depending on your symptoms and the specific heart condition, your doctor may prescribe a different type of pacemaker.
Use our list of questions to help guide a conversation with your doctor.
What is a leadless pacemaker?
A leadless pacemaker is a small, wire-free device implanted directly into the heart to regulate its rhythm. It is implanted in your heart through a blood vessel in your leg.
What is the difference between a leadless and traditional pacemaker?
Unlike traditional pacemakers, leadless pacemakers do not require electrical leads (wires) threaded through veins. Leadless pacemakers are 10 times smaller than traditional models. This design reduces complications and provides a minimally invasive implantation procedure.
Are pacemakers reliable?
Yes, pacemakers are generally considered safe and effective. They have a long history of successfully treating heart rhythm disorders and improving the quality of life for many people.
How is a leadless or traditional pacemaker implanted?
Leadless pacemakers are inserted via a catheter through a small incision in your groin and attached directly to a wall inside the heart through a minimally invasive procedure. Traditional pacemakers are surgically implanted under the skin in the chest area and connected to leads that attach to the heart wall.
What restrictions exist with a pacemaker?
With a traditional pacemaker, normal activity can resume around six weeks after implantation. Some arm movement restrictions will exist. Normal activity can resume around two weeks after implantation of a leadless pacemaker, and there are no arm movement restrictions.
Remote Care with Your Pacemaker
Remote monitoring allows your pacemaker to share information about your heart directly with your doctor’s office
without having to go in-person.
Some pacemakers use wireless remote monitoring, while others need a wand or skin electrodes for
communication.
The Merlin@home™ transmitter syncs with your traditional pacemaker with leads and the AVEIR™ Patient
Transmitter syncs with your leadless pacemaker. Both transmitters collect data on your heart’s activity, including
heart rate and rhythm, and shares it with your doctor for monitoring.
References
- AVEIR Leadless Pacemakers and Delivery Catheter IFU. ARTEN600361108.
- Assurity MRI Pacemaker IFU. ARTEN600226857_A.
Indications, Safety & Warnings
Rx Only
Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
Indications for Use: The AVEIR™ Leadless Pacemaker system is indicated for management of one or more of the following chronic clinical presentations: syncope, pre-syncope, fatigue, disorientation, and one or more of the indications which follow. Rate-modulated pacing is indicated for patients with chronotropic incompetence, and for those who would benefit from increased stimulation rates concurrent with physical activity. Dual-chamber pacing is indicated for patients exhibiting one or more of the following conditions: sick sinus syndrome; chronic, symptomatic second- and third-degree AV block; recurrent Adams-Stokes syndrome; symptomatic bilateral bundle-branch block when tachyarrhythmia and other causes have been ruled out. Atrial pacing is indicated for patients with: sinus node dysfunction and normal AV and intraventricular conduction systems. Ventricular pacing is indicated for patients with significant bradycardia and: normal sinus rhythm with only rare episodes of AV block or sinus arrest, chronic atrial fibrillation. MR Conditional: The AVEIR Leadless Pacemaker is conditionally safe for use in the MRI environment and according to the instructions in the MRI-Ready Leadless System Manual.
Intended Use: The AVEIR™ Leadless Pacemaker (LP) is designed to provide bradycardia pacing as a pulse generator with built-in battery and electrodes for implantation in the right ventricle and the right atrium. The LP is intended to provide sensing of intrinsic cardiac signals and delivery of cardiac pacing therapy within the implanted chamber for the target treatment group. The LP is also intended to operate optionally with another co-implanted LP to provide dual-chamber pacing therapy.
The AVEIR™ Delivery Catheter is intended to be used in the peripheral vasculature and the cardiovascular system to deliver and manipulate an LP. Delivery and manipulation includes implanting an LP within the target chamber of the heart.
Contraindications: Use of the AVEIR™ Leadless Pacemaker is contraindicated in these cases:
Use of any pacemaker is contraindicated in patients with a co-implanted ICD because high-voltage shocks could damage the pacemaker and the pacemaker could reduce shock effectiveness.
Single-chamber ventricular demand pacing is relatively contraindicated in patients who have demonstrated pacemaker syndrome, have retrograde VA conduction, or suffer a drop in arterial blood pressure with the onset of ventricular pacing.
Programming of rate-responsive pacing is contraindicated in patients with intolerance of high sensor driven rates.
Use is contraindicated in patients with an implanted vena cava filter or mechanical tricuspid valve because of interference between these devices and the delivery system during implantation.
Persons with known history of allergies to any of the components of this device may suffer an allergic reaction to this device. Prior to use on the patient, the patient should be counseled on the materials (listed in the Product Materials section of the IFU) contained in the device and a thorough history of allergies must be discussed.
Adverse Events: Potential complications associated with the use of the AVEIR™ Leadless Pacemaker system are the same as with the use of single or dual chamber pacemakers with active fixation pacing leads including, but not limited to: cardiac perforation; cardiac tamponade; pericardial effusion; pericarditis; endocarditis; thrombus formation; thromboembolism; valve damage or regurgitation; heart failure; pneumothorax/hemothorax; cardiac arrhythmias; diaphragmatic/phrenic nerve stimulation / extra-cardiac stimulation; palpitations; hypotension; syncope; cerebrovascular accident; infection; hypersensitivity reaction to device materials, contrast media, medications, or direct toxic effect of contrast media on kidney function; pacemaker syndrome; inability to interrogate or program the LP due to programmer or LP malfunction; intermittent or complete loss of capture, pacing or sensing (non-battery related); oversensing; increased capture threshold; inappropriate sensor response; corrupted, intermittent, or loss of i2i communications; interruption of desired LP function due to electrical interference, either electromyogenic or electromagnetic; battery malfunction/ premature battery depletion; device-related complications (premature deployment, device dislodgement/embolization of foreign material, inability to release/re-dock of the LP from catheter, helix distortion); additional surgery or intervention; death. As with any percutaneous catheterization procedure, potential complications include, but are not limited to: vascular access complications, such as perforation, dissection, puncture, groin pain; bleeding or hematoma; thrombus formation; thromboembolism; air embolism; local and systemic infection; peripheral nerve damage; general surgery risks and complications from comorbidities; such as dyspnea, respiratory failure, pneumonia, hypertension, cardiac failure, reaction to sedation, renal failure, anemia, and death.
Refer to the User’s Manual for detailed indications, contraindications, warnings, precautions, and adverse events.
™ Indicates a trademark of the Abbott group of companies.
‡ Indicates a third-party trademark, which is property of its respective owner.
MAT-2105184 v11.0
