Clinical Evidence for Stealth 360™ Peripheral Orbital Atherectomy System
The Stealth 360™ Peripheral Orbital Atherectomy System (OAS) has been well-studied in over 4,800 patients and in more than 7,000 lesions with durable results.1 In the LIBERTY 360 trial, primary patency was 89.7% at 2 years in RC 2-3 patients2 and the 3-year freedom from major amputation was 88.6% in RC 6 patients.3
Stealth 360™ Peripheral OAS Clinical Studies
LIBERTY 360º
LIBERTY is the largest, contemporary, real-world study to evaluate procedural and long-term clinical outcomes of endovascular device interventions in patients with symptomatic lower extremity Peripheral Artery Disease (PAD).4
Study Design4
- Prospective, observational, multicenter study that includes any FDA-cleared or approved technology to treat claudication and Critical Limb Ischemia (CLI).
- 1,204 patients enrolled at 51 sites and were followed for up to five years. Four core labs were used for independent analysis.
- Key endpoints: Procedural and lesion success, Major Adverse Events (MAEs), Duplex Ultrasound (DUS), Quality of Life (QoL), and Six-Minute Walk Test (6MWT).
- Key inclusion criteria: Rutherford classification (RC) 2 to 6, target lesion located within or extending into 10 cm above the medial epicondyle to the digital arteries (distal 1/3 of the SFA and below), and at least one lesion that can be treated with an endovascular device.5

Conclusion
Peripheral Vascular Intervention (PVI) may be a reasonable treatment option across all Rutherford classes with durable results lasting to 3 years.4
- High freedom from major amputation at 3 years across all Rutherford classes.4
- In the OAS sub-analysis, freedom from major amputation rates at 3 years were even higher than in the full patient cohort.4
- Improved Quality of Life by 30 days and maintained to 3 years across all Rutherford classes.4
- High long-term patency rates through 2 years in RC 2-3.6
- Improvement in mean number of wounds from 30 days to 2 years in RC 4-6.6
CALCIUM 360º
A prospective, randomized, multi-center study that compared the acute and long-term results of Orbital Atherectomy System (OAS) + Percutaneous Transluminal Angioplasty (PTA) versus PTA alone in calcified below-the-knee (BTK) lesions.7
Key Takeaways7
- Lower mean maximum balloon inflation pressure in OAS+PTA arm
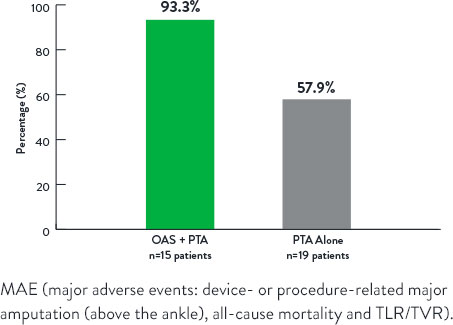
- Lower rate of major adverse events at 12-months in OAS+PTA arm
Study Design7
- 50 Critical Limb Ischemia (CLI) patients enrolled at 8 U.S. sites.
- Patients randomized to either OAS+PTA (25 pts) or PTA alone (25 pts) and followed for 12 months.
Patient Population7
| Demographics | OAS + PTA (n=25) | PTA Alone (n=25) | p-value |
|---|---|---|---|
| Mean Age | 70.7 ± 13.4 | 71.8 ± 10.9 | 0.75 |
| Male/Female | 68%/32% | 60%/40% | 0.77 |
| Diabetic Type 1 | 4% | 0% | 1.00 |
| Diabetic Type 2 | 68% | 56% | 0.56 |
| Renal Insufficiency (GFR <90) | 25% | 24% | 1.00 |
| Smoker (current or previous) | 60% | 60% | 1.00 |
| CAD | 44% | 56% | 0.57 |
| Hypertension | 84% | 84% | 1.00 |
| Dyslipidemia | 83% | 72% | 0.50 |
Study Results7
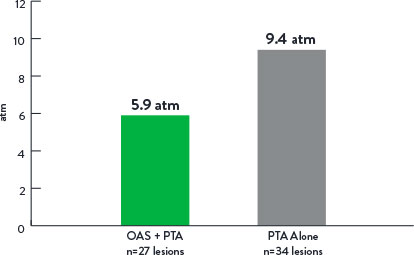
Mean Maximum Balloon Inflation Pressure (atm)
p=0.001
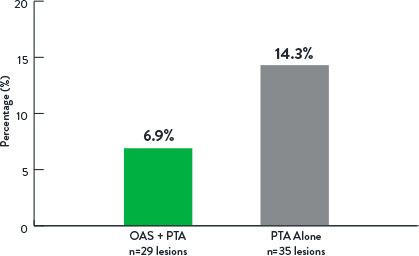
Rate of Bail-Out Stenting
p=0.44
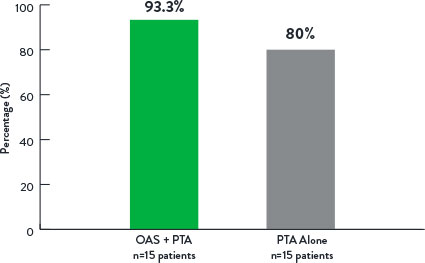
Freedom From Revascularization at 12 Months
p=0.14
Freedom From Major Adverse Events at 12 Months
p=0.006
TRUTH
A prospective, single-arm, single-center study using Intravascular Ultrasound (IVUS) to assess Orbital Atherectomy System (OAS)-related plaque modification of femoropopliteal lesions.8
Key Takeaways8
- OAS modified the calcified component of the plaque burden (IVUS analysis).
- Calcium reduction was responsible for 86% of lumen area increase (IVUS analysis).
- Increase in minimum lumen area post-OAS (IVUS analysis).
- Low mean maximum balloon inflation pressure post-OAS of 5.2 atm.
Study Design8
- 25 patients enrolled at a single center.
- Patients treated with OAS+Percutaneous Transluminal Angioplasty (PTA) and followed for 12 months.
- IVUS images collected pre-OAS, post-OAS, and post-PTA (IVUS Core Lab).
Patient Population8
| Baseline Characteristics | (n=25) |
|---|---|
| Age | 70.4 ± 7.8 years |
| Gender (Male) | 19/25 (76.0%) |
| eGFR (mL/min/1.73 m2) | 70.9 ± 25.0 |
| History of diabetes (Type I or II) | 18/25 (72.0%) |
| History of hyperlipidemia | 25/25 (100.0%) |
| History of hypertension | 25/25 (100.0%) |
| Smoker (current or former) | 21/25 (84.0%) |
| Rutherford classification | 3.0 ± 0.0 |
Study Results8
- The mean maximum balloon inflation pressure was 5.2±1.2 atm.
- Virtual histology IVUS analysis revealed at the maximum calcium ablation site that calcium reduction was responsible for 86% of the lumen area increase.
- The minimum lumen area increased from 4.0 mm2 to 9.1 mm2 (P<0.0001).
| Rutherford Classification (RC) | Baseline (n=25) | 12-Month Follow-up (n=22) |
|---|---|---|
| Asymptomatic (RC 0) | 0 (0.0%) | 13 (59.1%) |
| Mild Claudication (RC 1) | 0 (0.0%) | 8 (36.4%) |
| Moderate Claudication (RC 2) | 0 (0.0%) | 1 (4.5%) |
| Severe Claudication (RC 3) | 25 (100.0%) | 0 (0.0%) |
| ABI* | 0.74 ± 0.13 (n=22) | 0.95 ± 0.15 (n=21) |
*Greater of posterior tibial or dorsalis pedis systolic pressure divided by maximum of left or right brachial systolic pressure.
p-value <0.001 for Rutherford Classifications and ABI
At 12 months, the freedom from target lesion revascularization rate was 91.8%, and ankle-brachial index and Rutherford classification improved significantly from baseline through follow-up.
COMPLIANCE 360°
A prospective, randomized, multi-center study that compared the acute and long-term results of Orbital Atherectomy System (OAS) + Percutaneous Transluminal Angioplasty (PTA) versus PTA alone in calcified above-the-knee (ATK) lesions.9
Key Takeaways9
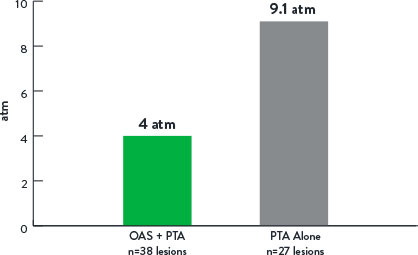
- Lower mean maximum balloon inflation pressure in OAS+PTA arm.
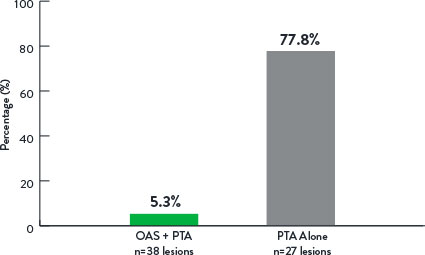
- Lower adjunctive stent rate in OAS+PTA arm.
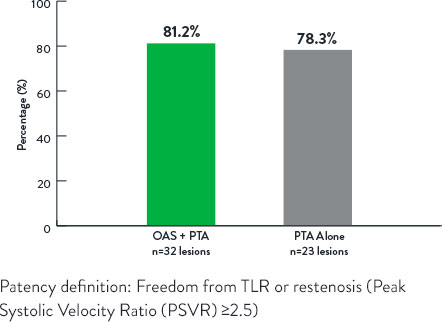
- OAS demonstrated reduced stenting with durable results out to 12 months versus PTA alone.
Study Design9
- 50 patients enrolled at 9 U.S. sites.
- Patients randomized to either OAS+PTA (25 pts) or PTA alone (25 pts) and followed for 12 months.
Patient Population9
| Comorbidity | OAS + PTA (n=25) | PTA Alone (n=25) | p-value |
|---|---|---|---|
| Mean Age ± SD | 68.0 ± 11.0 | 71.3 ± 10.5 | 0.27 |
| Female gender, n (%) | 18 (72) | 16 (64) | 0.76 |
| Non-Caucasian, n (%) | 9 (36) | 4 (16) | 0.19 |
| Coronary artery disease, n (%) | 12 (48) | 16 (64) | 0.39 |
| Diabetes, n (%) | 18 (72) | 10 (40) | 0.05 |
| Hypertension, n (%) | 22 (88) | 18 (72) | 0.29 |
| Hyperlipidemia, n (%) | 23 (92) | 21 (84) | 0.67 |
| Smoker (current or former), n (%) | 22 (88) | 22 (88) | >0.99 |
Study Results9
Mean Maximum Balloon Inflation Pressure (atm)
p<0.001
Adjunctive Stenting
p<0.001
Patency at 12 Months
p<0.99
References
- Data on file at Abbott. Counts updated 06Jan2020 - subject to change (includes PAD I, PAD II, OASIS, CONFIRM, CALCIUM, COMPLIANCE, TRUTH, CLARITY, LIBERTY, OPTIMIZE, REACH PVI).
- Adams GL, et al. J Vasc Surg. 2019;70(5 Suppl):E188-E189.
- Giannopoulos S, et al. J Endovasc Ther. 2020;27(5):714-725.
- Mustapha JA. LIBERTY 360 Trial 3-Year Update. Presented at AMP 2019.
- Adams GL, et al. Am Heart J. 2016;174:14-21.
- Mustapha JA. Late Breaking: LIBERTY 360 2-Year Update. Presented at AMP 2018.
- Shammas NW, et al. J Endovasc Ther. 2012;19(4):480-488.
- Babaev A, et al. Vasc Endovasc Surg. 2015;49(7):188-194.
- Dattilo R, et al. J Invasive Cardiol. 2014;26(8):355-360.
MAT-2402041 v1.0
