
Rapid Exchange (RX) platform
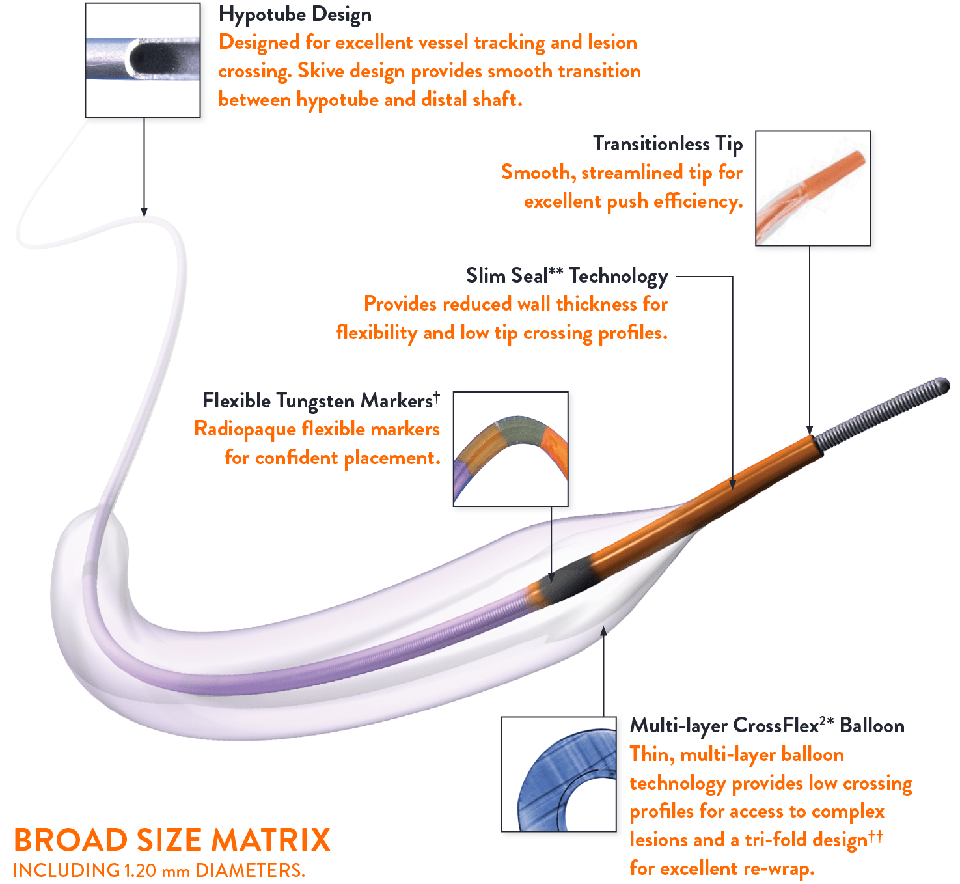
Transitionless Design for Challenging Anatomy
TREK™ Coronary Dilatation Catheter’s unique design provides smooth transitions from hub to tip.
- Transitionless tip
- Flexible distal shaft
- Multi-layer CrossFlex²* balloon and Slim Seal** technology
Mini Trek™ Coronary Dilatation Catheter Indicated for Treatment of De Novo Chronic Total Occlusions
MINI TREK™ Coronary Dilatation Catheter’s ultra low profile design enables lesion access.
- Small chassis designed distinctively for MINI TREK™ Coronary Dilatation Catheter
- Available in sizes as small as 1.20 mm
Technical Information
| Trek™ & Mini Trek™ Coronary Dilatation Catheters | |
|---|---|
| Nominal Pressure (NP) | 8 atm |
| Rated Burst Pressure (RBP) | 14 atm |
| Tip Entry Profile | 0.017 in (3.00 mm Ø) |
| Tip Crossing Profile | 0.021 in average (3.00 mm Ø) 0.023 in maximum |
| Folded Balloon Crossing Profile | 0.032 in average (3.00 mm Ø) 0.036 in maximum (3.00 mm Ø) |
| Refolded Balloon Crossing Profile | 0.056 in maximum (3.00 mm Ø) |
| RX Notch Diameter (For RX Platform) | 2.5 French average (3.00 mm Ø) 2.7 French maximum (all Ø) |
| Tip Length | <3 mm |
| Balloon Material | Pebax‡, semi-compliant |
| Marker Material | Flexible Tungsten/Pebax‡ |
| Catheter Length | 145 cm |
| Balloon Coating | Hydrophilic |
| Minimum Guide Catheter | 5 French (1.20 mm – 4.00 mm Ø) 6 French (4.50 mm & 5.00 mm Ø) |
| Maximum Guide Wire Compatibility | 0.014 in |
| Latex Free | Yes |

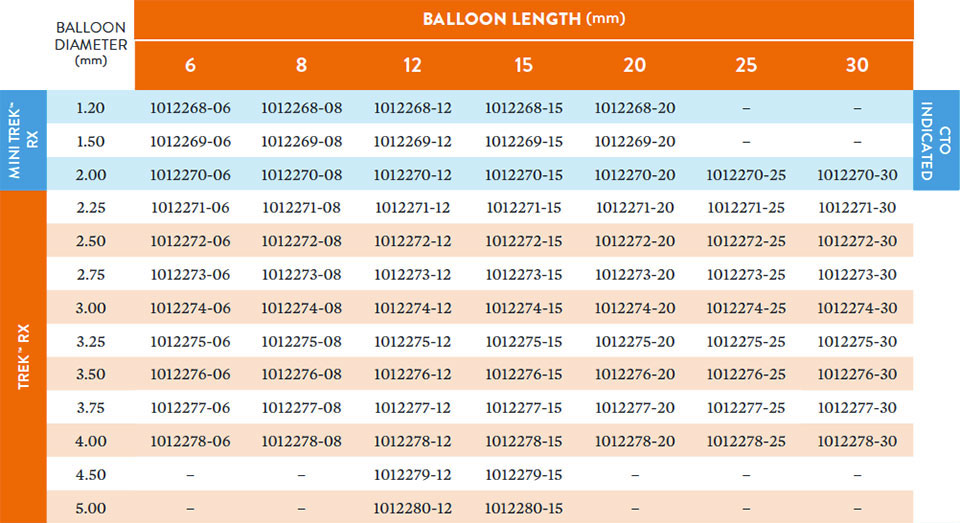
Ordering Information
Broad Size Matrix with 77 Sizes
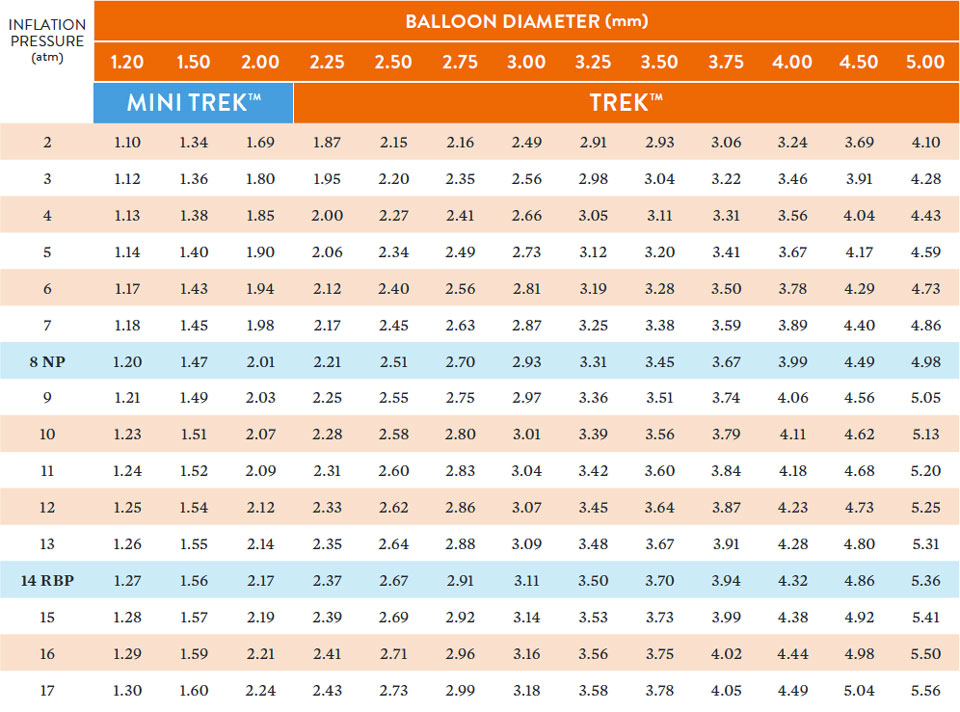
Compliance Chart
Data on file at Abbott.
**Slim Seal technology available on 2.0 mm – 3.75 mm balloon sizes.
† Single marker on all 6 mm lengths and 1.20 – 1.50 mm diameters (all lengths). Dual markers on all other sizes.
*CrossFlex2 technology available on 2.25 mm – 5.0 mm balloon sizes.
††Tri-fold design for 2.00 mm – 4.00 mm diameters. Bi-fold design for 1.20 mm & 1.50 mm diameters.
MAT-2111436 v2.0
