Patient Selection & Assessment
Selecting the Right Patient
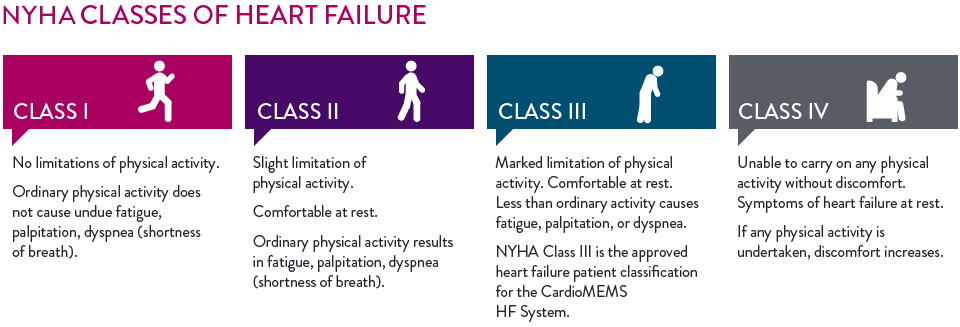
The CardioMEMS™ HF System is indicated for patients with:
- NYHA Class III heart failure; and
- One heart failure hospitalization in the past 12 months
The CardioMEMS HF System is contraindicated for patients with:
- An inability to take dual antiplatelet or anticoagulants for one-month post implant
Patients who most commonly receive the CardioMEMS HF System are those on GDMT and those who exhibit any of the following:
- Fluid volumes that are hard to know or manage
- Challenging physical assessment
- Is a patient with HFpEF or HFrEF
- Compliant with heart failure medical care
- Would benefit from remote monitoring if they live far from a clinic
Note: The CHAMPION trial specifically excluded patients with American College of Cardiology/ American Heart Association stage D heart failure who needed advanced therapies (i.e., left ventricular assist device, transplant or inotropic support). Even if inotropic support improved heart failure symptoms, a patient would still be defined as stage D, with refractory heart failure.1
This device is commercially available for use in select international markets.
References
- Abraham, W. T., Adamson, P. B., Bourge, R. C., Aaron, M., Costanzo, M. R., Stevenson, L. W., …Yadav, J. S., for the CHAMPION Trial Study Group. (2011). Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. The Lancet, 377(9766), 658-666. https://dx.doi.org/10.1016/S0140-6736(11)60101-3
MAT-2116103 v4.0