Pioneering Innovation in LBBAP
Experience the innovation of the UltiPace Pacing Lead, Abbott's latest breakthrough, and the only approved stylet-driven lead for left bundle branch area pacing (LBBAP) with a robust distal lead tip design.* Engineered for superior performance in the pursuit of continuous innovation.1

Proven Design Evolved
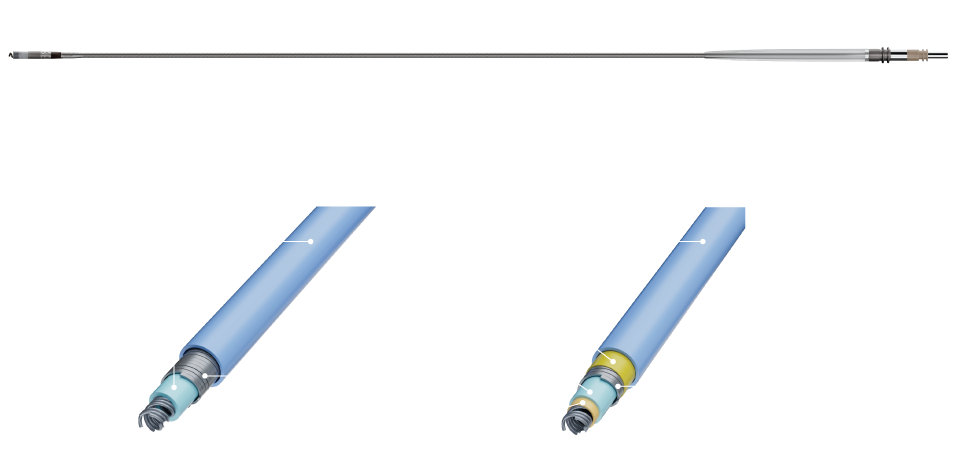
vs. Tendril™ STS1
3X
Increase in abrasion resistance
3.5X
Increase in lead crush resistance
vs. Tendril MRI™ 1
2.2X
Increase in abrasion resistance
2.6X
Increase in lead crush resistance
Demonstrated LBBAP Success and Safety
Composite
success
rate2**
of patients not experiencing
LBBAP-related adverse effects at 6 months post-implant.2
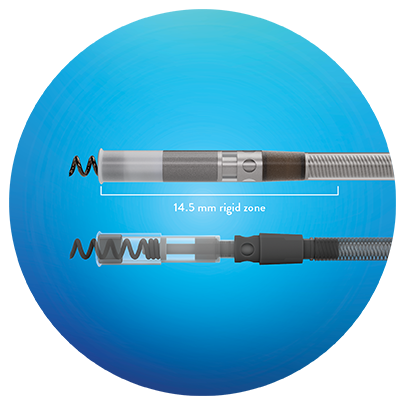
Resistant to Distal Fatigue Failure
UltiPace Pacing Leads have a robust distal lead tip without a flexible zone that helps reduce stress on the conductors and the chance for distal fatigue failure compared to competitive leads.3
Zero Helix Retraction
The Helix Locking Tool is a proven solution to lock an extended helix during tissue burrowing without retraction.3
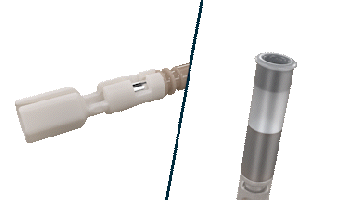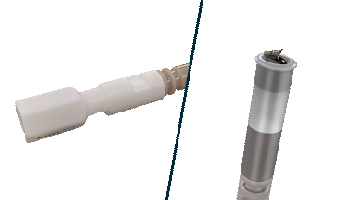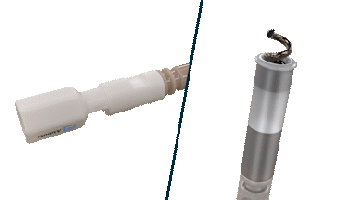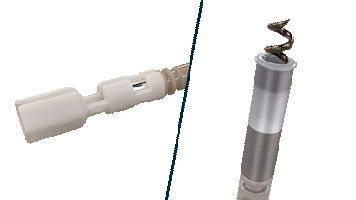
The Helix Locking Tool aids with lead fixation by providing control over the extension and retraction of the helix.
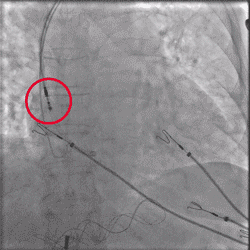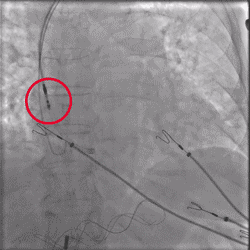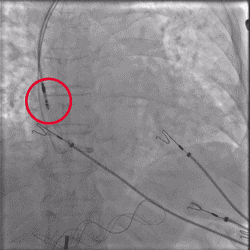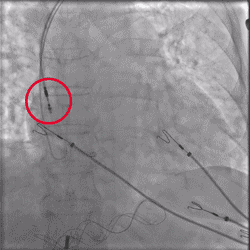
Improved Torque Transmission with Fewer Rotations
Improvement in torsional stiffness when using UltiPace Pacing Leads compared to LBBAP-approved lumenless pacing leads.3
Torque being applied to UltiPace Pacing Lead body in LBBAP procedure.
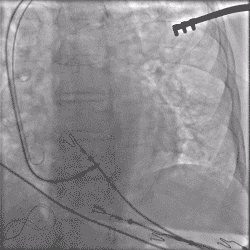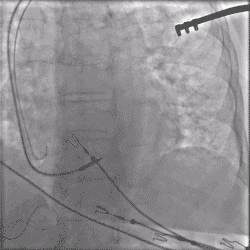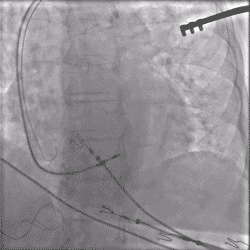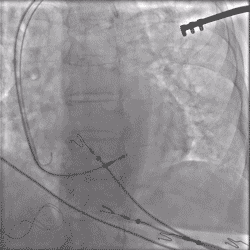
Continuous Unipolar Pacing and Impedance Monitoring
Provides the user with uninterrupted feedback to help achieve optimal results.4
References
*Robust distal lead tip without a flexible zone in distal 14.5 mm, CE and FDA Approval for LBBAP.
**Composite success rate of acceptable capture thresholds and sense amplitudes for LBBAP at 6 months post-implant.
- Abbott. Data on File. Item: 91010074 Ver A.
- Abbott. Data on File.
- Abbott. Data on File, Engineering Technical Report. Item: 90994484 Ver D.
- Burri, H., Jastrzebski, M., Cano, O., Curila, K., Pooter, J. de, Huang, W., Israel, C., Joza, J., Romero, J., Vernooy, K., Vijayaraman, P., Whinnett, Z., & Zanon, F. (2023). EHRA clinical consensus statement on conduction system pacing implantation: Executive summary. endorsed by the Asia-Pacific Heart Rhythm Society (APHRS), Canadian heart rhythm society (CHRS) and Latin-American heart rhythm society (LAHRS). Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. https:// pubmed.ncbi.nlm.nih.gov/37061850/
Indications:
The UltiPace™ leads are indicated for use in combination with a compatible pacemaker, implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy (CRT ‑P/CRT ‑D) device to provide sensing and pacing for the management of chronic symptomatic bradycardia and various atrioventricular conduction abnormalities in patients who experience syncope, presyncope, fatigue, or disorientation due to arrhythmia/bradycardia, or any combination of these symptoms. The UltiPace leads are implanted transvenously in either the right atrium, the right ventricle or the left bundle branch area.
For indications for the pacing system, please refer to the applicable pulse generator manual.
MAT-2411896 v2.0

