Index of Microcirculatory Resistance (IMR) and Coronary Flow Reserve (CFR) for Microvascular Assessment
Among stable patients undergoing cardiac catheterization, the majority (59%) have little or no angiographic abnormality, as shown in the visual1 in spite of the fact that the majority have symptoms of a coronary disorder.2 Patients with ischemia and no obstructive artery disease (INOCA) may have coronary microvascular dysfunction (CMD). As a group, these patients are underdiagnosed.
The index of microcirculatory resistance (IMR) and coronary flow reserve (CFR) are used to assess the microvasculature to help provide a diagnosis of CMD.
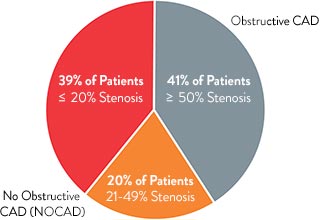
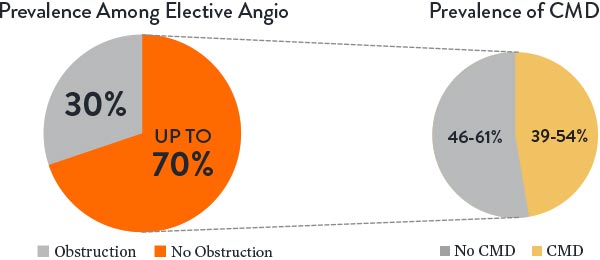
Estimated Prevalence of INOCA and CMD3
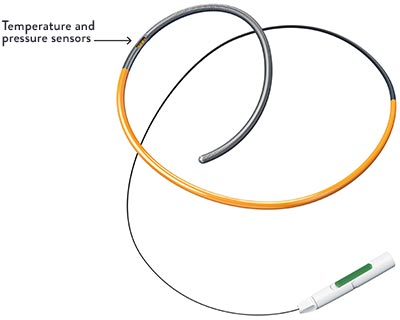
IMR and CFR are calculated from the two temperature sensors located in proximal and distal positions on the PressureWire™ X Guidewire.4,5 These sensors allow physicians to capture the measurements using thermodilution.
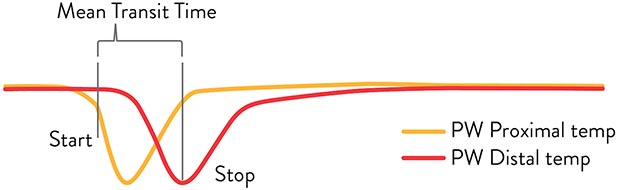
Using Thermodilution to Evaluate IMR and CFR6
When an interventional cardiologist (IC) injects saline flush at ambient temperature into the artery, the PressureWire™ X Guidewire detects temperature changes as the saline passes the proximal and distal sensors.
Coronary flow is estimated based on the time it takes the saline to pass between proximal and distal sensors. This time (in seconds) is known as the Mean Transit Time (Tmn).
Learn How to Assess and Diagnose CMD by Following the CATH CMD Algorithm
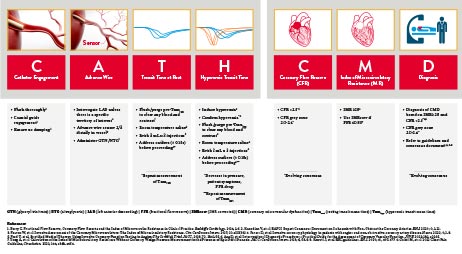



The CoroFlow‡ Cardiovascular System can be used to review physiology measurements.7
IMR = measures the blood flow in microvasculature
CFR = measures the blood flow in the epicardial arteries and the microvasculature
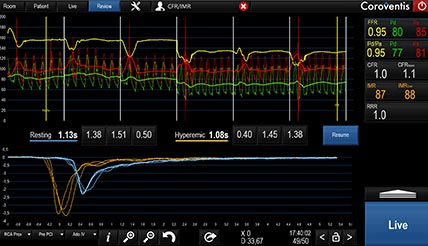
Diagnosing Coronary Microvascular Dysfunction
IMR and CFR cutoffs in a population of INOCA patients are shown on the right (based on CorMicA trial).8
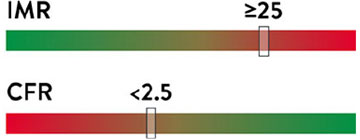
Cutoff values are specific for INOCA patients
Why Assess for Coronary Microvascular Dysfunction?
INOCA patients may be suffering from persistent angina due to CMD and are at higher risk of major adverse cardiac events (MACE).9 Until such symptomatic patients receive proper treatment, they are frequent consumers of healthcare resources due to repeat evaluations, cath lab tests, emergency room visits, and hospitalizations.10-12
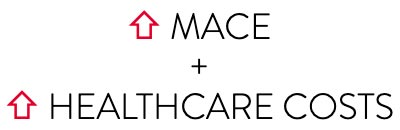
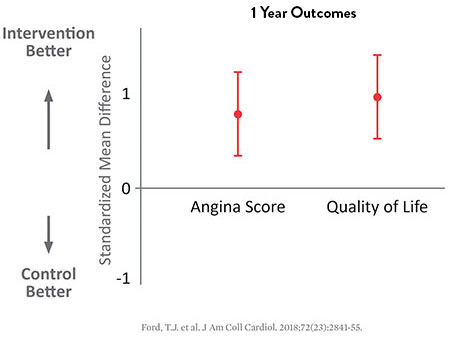
As the CorMicA study reveals, patients may benefit when coronary microvascular dysfunction is accurately diagnosed and properly treated.8,13
27% Improvement in Angina Severity†
18% Improvement in Quality of Life
In addition, a comprehensive physiology assessment was demonstrated to be highly cost-effective at £4,500 per QALY. This is well below the accepted cost-effectiveness threshold of £20,000 per QALY for NICE (UK).14-16
Proper assessment, diagnosis, and treatment of CMD can improve outcomes in CMD patients at high risk for MACE and reduce healthcare burden.3,9,17
Following Society Guidelines for IMR and CFR
ESC and ACC/AHA guidelines recommend measuring IMR and CFR using a guidewire-based approach for symptomatic patients who exhibit no significant evidence of epicardial stenosis.
The ESC guidelines18 2019 were updated accordingly to include an increased focus on microvascular dysfunction.
| Recommendations | Classa | Levelb |
|---|---|---|
| Guidewire-based CFR and/or microcirculatory resistance measurements should be considered in patients with persistent symptoms, but coronary arteries that are either angiographically normal or have moderate stenosis with preserved iwFR/FFR | IIa | B |
The AHA/ACC Clinical Practice Guideline on chest pain includes Class IIa recommendation for guidewire-based assessment for INOCA patients.19
| Recommendations for Patients With INOCA | Classa | Levelb |
|---|---|---|
| For patients with persistent stable chest pain and non-obstructive CAD and at least mild myocardial ischemia on imaging, it is reasonable to consider invasive coronary function testing to improve the diagnosis of coronary microvascular dysfunction and to enhance risk stratification. | IIa | B-NR |
CFR = coronary flow reserve; CMR = cardiac magnetic resonance; ECG = electrocardiogram;
FFR = fractional flow reserve; iwFR = instantaneous wave-free ratio; LAD = left anterior descending; PET = positron emission tomography.
a Class of recommendation.
b Level of evidence.
Level-NR: Level (Quality) of Evidence Level B-NR (non-randomized): moderate-quality evidence from 1 or more well designed, well executed non-randomized studies, observational studies or registry studies. RCTs. Meta-analyses of such studies.
The PressureWire™ X Guidewire with CoroFlow‡ Cardiovascular System has the capability to wirelessly measure comprehensive physiology indices to assess for epicardial disease (FFR, RFR) and microvascular dysfunction (IMR, CFR).4,7
References
- Patel, MR., et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886-895. doi:10.1056/NEJMoa0907272.
- Ford, TJ., et al. How to diagnose and manage angina without obstructive coronary artery disease: lessons from the British Heart Foundation CorMicA Trial. Interv Cardiol Rev. 2019;14(2):76-82.
- Kunadian V, et al. EAPCI Expert Consensus Document. Eur Heart J 2020;0:1-21.
- PressureWire™ Guidewire Instructions for Use (IFU). Refer to IFU for additional information.
- Fearon and Kobayashi, Invasive assessment of the coronary microvasculature, CCI 2017; 10:e005361.
- DeBruyne, et al. Coronary Thermodilution to Assess Flow Reserve Experimental Validation. Circulation. 2001;104:2003-2006.
- CoroFlow‡ Cardiovascular System Instructions for Use (IFU). Refer to IFU for additional information.
- Ford, TJ., et al. 1-year outcomes of angina management guided by invasive coronary function testing (CorMicA). J Am Coll Cardiol Intv. 2020;13:33-45.
- Taqueti, VR., et al. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2625–2641. doi:10.1016/j.jacc.2018.09.042.
- Rahman, H., et al. Diagnosis of patients with angina and non-obstructive coronary disease in the catheter laboratory. Heart. 2019;105:1536-1542.
- Lee, B., et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060.
- Reriani, M., et al. Coronary endothelial function testing may improve long-term quality of life in subjects with microvascular coronary endothelial dysfunction. Open Heart. 2019;6:e000870. doi: 10.1136/openhrt-2018-000870.
- Ford, TJ., et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72:2841-2855.
- Heggie R et al. Stratified medicine using invasive coronary function testing in angina: a cost-effectiveness analysis of the British Heart Foundation CorMicA trial. IJC. 2021; doi: 10.1016/j.ijcard.2021.05.016.
- Quality Adjusted Life Year (QALY) is a measure that consists of quality of life and length of life.
- Dubois R. Cost-effectiveness threshold in the US. JCER. 2016(5); doi:10.2217/cer.15.50. <50,000 USD/QALY is high value.
- Jespersen, L., et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734-744. doi:10.1093/eurheartj/ehr331.
- Knuuti, J., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal 2019.
- Gulati, M., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines.
MAT-2211668 v1.0
