Unmatched 1-Year Patency & 3-Year Freedom From TLR
The Supera™ Stent has been studied in over 2,000 patients worldwide in the SUPERB trial and 16 retrospective studies. Notably, in all of the 17 studies, the Supera™ Peripheral Stent showed durable results with zero fractures at 1 year.1,15-30
SUPERB Trial
At 1 year the Supera™ Stent demonstrated primary patency of 91% when nominally* deployed. At 3 years, freedom from targeted lesion revascularization (TLR) was 94% when nominally* deployed.1
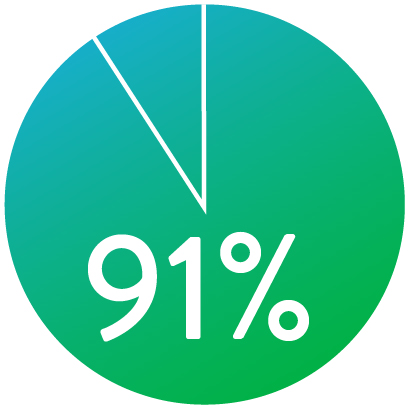
PATENCY (K-M) AT 1 YEAR
When nominally deployed*
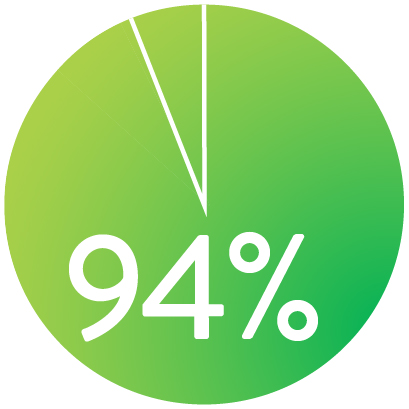
FREEDOM FROM TLR AT 3 YEARS
When nominally deployed*
*Nominal deployment is defined as the stent length upon deployment being within +/- 10% of the labeled stent length. This data is from a non-powered post-hoc analysis. K-M=Kaplan Meier.
Unmatched Clinical Outcomes
Supera Stent has demonstrated unmatched clinical outcomes in comparison to other US pivotal trial results which primarily included simple lesions.*,1-12
| 1-Year Patency (KM) | 3-Year Freedom from TLR | |||
|---|---|---|---|---|
| 91% | Supera™ Stent Nominal1 | 94% | Supera™ Stent Nominal1 | |
| 87% | Eluvia‡12 | Not Reported | Eluvia‡ | |
| 84% | Zilver PTX‡2 | 84% | Zilver PTX‡2 | |
| 83% | Misago‡11 | Not Available | Misago‡ | |
| 81% | LifeStent‡3 | 76% | LifeStent‡4 | |
| 80% | S.M.A.R.T.‡5 | 79% | S.M.A.R.T.‡6 | |
| 77% | EverFlex‡7 | 70% | EverFlex‡8 | |
| 74% | Innova‡9 | Not Reported | Innova‡ | |
| 67% | Pulsar‡10 | Not Reported | Pulsar‡ | |
* Study reported 93.8% with Trans-Atlantic Inter-Society Consensus Document (TASC) A & B lesions and/or Rutherford class 2 or 3 lesions
NOTE: Results from clinical trials are not directly comparable. Information provided for educational purposes only.
Consistent Patency Regardless of Lesion Length
With some peripheral stents, increasing lesion lengths can lead to decreasing patency rates.31 The Supera™ Stent stands apart for its consistently high patency rates in lesions spanning lengths from 5.3 cm up to 28.0 cm.†
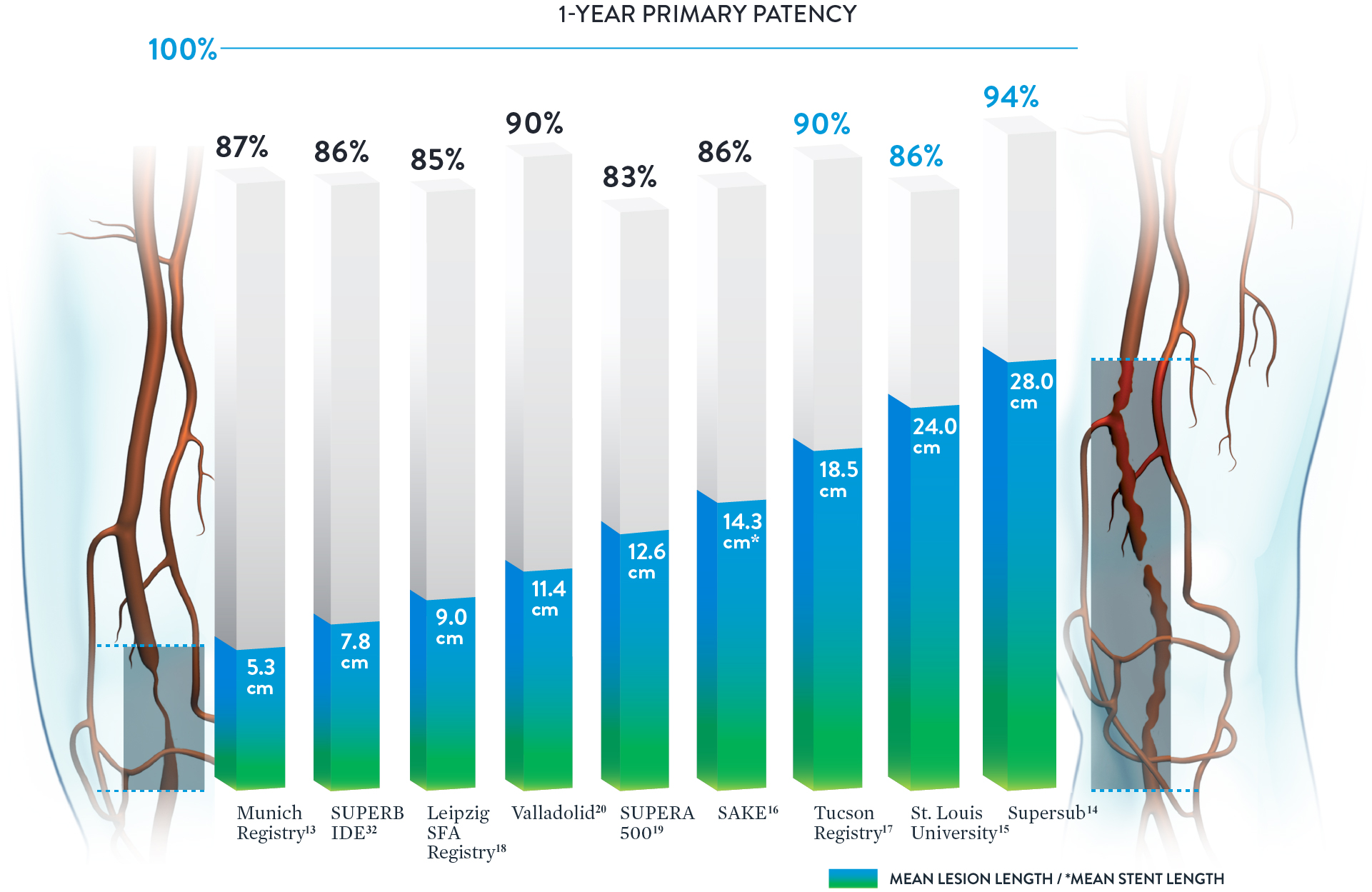
Excellent Results from Simple to Complex Lesions
Whether treating simple (TASC A&B) or complex (TASC C&D) lesions, the Supera™ Stent is associated with impressive, consistent patency performance data at 1 year.13-15,32
 | Trial/Study | MUNICH REGISTRY13 | SUPERB32 |
|---|---|---|---|
| Lesion Length | 5.3cm | 7.8cm | |
| TASC A&B Lesions | 100% | 94% | |
| 1-Yr Patency | 86.7% | 90.5% | |
| Sites | Single Center | Multicenter (46 sites) | |
| # Patients | 70 | 264 | |
 | Trial/Study | ST. LOUIS15 | SUPERSUB14 |
|---|---|---|---|
| Lesion Length | 5.3cm | 7.8cm | |
| TASC C&D Lesions | 78% | 100% | |
| CTOs | Unknown | 100% | |
| 1-Yr Patency | 85.6% | 94.1% | |
| Sites | Single Center | Single Center | |
| # Patients | 48 | 34 | |
TASC: Trans-Atlantic Inter-Society Consensus
REFERENCES
- Garcia L. et al., Catheterization and Cardiovascular Interventions 2017 Jun 1;89(7):1259-1267.
- Dake M. et al., Circulation. 2016;133:1472-1483.
- Laird J. et al., Circ Cardiovasc Interv. 2010;3:267-276.
- Laird J et al., J Endovasc Ther. 2012;19:1–9.
- S.M.A.R.T. Control IFU.
- Jaff, M., SMART Nitinol Self-Expanding Stent in the Treatment of Obstructive Superficial Femoral Artery Disease: Three-year Clinical Outcomes from the STROLL Trial. ISET 2014.
- Matsumura J et al., J Vasc Surg 2013;58:73-83.
- Rocha-Singh, K., 3-Year Results of the DURABILITY II Study. VIVA 2013.
- US Innova IFU.
- US Pulsar IFU.
- Ohki T. et al. J Vasc Surg. 2016 Feb;63(2):370-6.
- Gray W. et al., Lancet 2018;392:1541-51.
- Treitl, K.M., et al. European Radiology.2017; 10.1007.
- Palena L.M. et al. Catheterization and Cardiovascular Intervention.2016.
- Brescia AA. et al., J Vasc Surg. 2015 Jun;61(6):1472-8
- George JC. et al., J Vasc Interv Radiol. 2014 Jun;25(6):954-61.
- Montero-Baker M. et al., J Vasc Surg. 2016 Oct;64(4):1002-8.
- Scheinert D. et al., J Endovasc Ther. 2011 Dec;18(6):745-52.
- Werner M. et al., EuroIntervention. 2014 Nov;10(7):861-8.
- San Norberto EM. et al., Ann Vasc Surg. 2017 May;41:186-195.
- Chan YC. et al., J Vasc Surg. 2015 Nov;62(5):1201-9.
- Dumantepe M. Vasc Endovascular Surg. 2017 Jul;51(5):240-246.
- Goltz JP. et al., J Endovasc Ther. 2012 Jun;19(3):450-6.
- León LR Jr. et al., J Vasc Surg. 2013 Apr;57(4):1014-22.
- Myint M. et al., J Endovasc Ther. 2016 Jun;23(3):433-41.
- Palena LM. et al., J Endovasc Ther. 2018 Oct;25(5):588-591.
- Scheinert D. et al., JACC Cardiovasc Interv. 2013 Jan;6(1):65-71.
- Steiner S. et al., J Endovasc Ther. 2016 Apr;23(2):347-55.
- Teymen B. et al., Vascular. 2018 Feb;26(1):54-61.
- Bhatt H. et al., Cardiovasc Revasc Med. 2018 Jul;19(5 Pt A):512-515.
- Shroë H. Superficial femoral artery PTA or stenting? 5-Year results. CIRSE 2011; Munich, Germany
- Garcia L. et al. Circ Cardiovasc Interv. 2015; 8:e00937.
MAT-2106532 v1.0
