Visit ClinicalTrials.gov
For more information about the LIFE-BTK study.
The LIFE-BTK (“below the knee”) study is evaluating Esprit™ BTK, a medical device manufactured by Abbott. Patient enrollment is complete. Esprit™ BTK is called a drug-eluting resorbable scaffold because it is placed in the artery immediately after a balloon angioplasty, supporting and preventing the vessel from reclosing. Once implanted, the scaffold releases a drug over a few months that promotes healing and keeps the artery open. The scaffold is naturally resorbed into the body over time, like dissolving stitches, and will be gone from your body in about 3 years, leaving only a healed artery behind.
The procedure is similar to the technique required to unblock arteries in the heart, but instead it is used below the knee to restore blood flow to the foot, with the goal of preventing amputation.
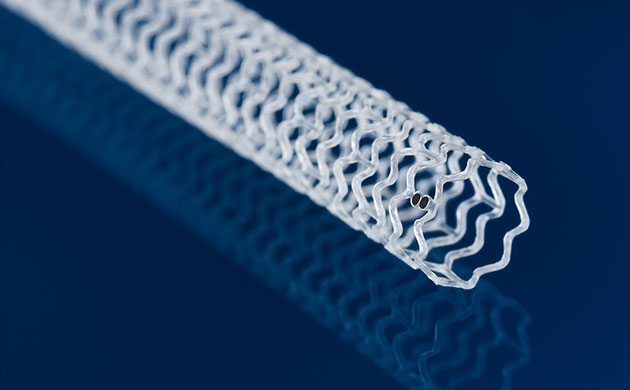
The purpose of the LIFE-BTK study is to find out whether this new treatment option could offer greater benefits than the current option of balloon angioplasty alone to open the blocked arteries in the leg and to keep the arteries open to prevent possible amputation.
Patients have enrolled across the U.S. For a detail listing of enrolled sites, go to clinicaltrials.gov