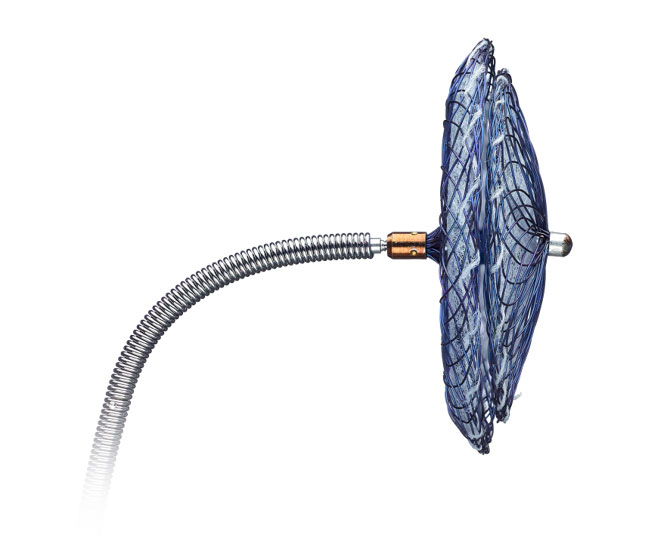
Effective PFO Closure Made Easier1
Over 25 years ago, we pioneered PFO closure with the Amplatzer™ PFO Occluder. Today, it is the most-studied — and most-trusted — device of its kind, with over 180,000 patients treated worldwide.2
And our clinical evidence is unmatched, thanks to the largest-ever trial for PFO closure, boasting 5,810 patient-years of data.3
With the introduction of the Amplatzer™ Talisman™ PFO Occluder, we have built upon the proven Amplatzer PFO closure technology. We've added the 30mm size so you have the complete PFO portfolio for every case. And the occluder now comes assembled and ready to use, simplifying prep and enhancing ease-of-use.
The easy-to-use just got easier.

Ordering Information
| Sizing and Device Selection | |||
|---|---|---|---|
| Amplatzer™ Talisman™ PFO Occluder | |||
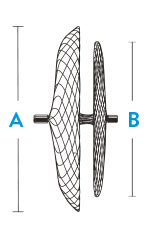
| Model/Reorder Number | Right Atrial Disc Diameter A | Left Atrial Disc Diameter B | Recommended Sheath Size |
| 9-PFO-1818 | 18 mm | 18 mm | 8 F; 45° curve |
| 9-PFO-2518 | 25 mm | 18 mm | 8 F; 45° curve |
| 9-PFO-3025 | 30 mm | 25 mm | 9 F; 45° curve |
| 9-PFO-3525 | 35 mm | 25 mm | 9 F; 45° curve |
| Delivery Sheath | |||||
|---|---|---|---|---|---|
| Amplatzer™ Talisman™ Delivery Sheath | |||||
| Model/Reorder Number | Sheath Size | Tip Angle | Sheath Inner Diameter | Sheath Outer Diameter | Usable Length |
| 9-TDS-08F45-80 | 8 F | 45° | 2.69 mm | 3.45 mm | 80 cm |
| 9-TDS-09F45-80 | 9 F | 45° | 3.00 mm | 3.81 mm | 80 cm |
| Ancillary Products | ||||
|---|---|---|---|---|
| Amplatzer™ Guidewire | ||||
| Model/Reorder Number | Diameter | Body | Tip Description | Usable Length |
| 9-GW-002 | 0.035 inch | Super Stiff | 1.55 mm, Modified J-tip | 260 cm |
| Device Sizing Guidelines | ||
|---|---|---|
| PFO Morphology | Example Anatomical Characteristics | Suggested Amplatzer™ Talisman™ PFO Occluder size (mm) |
| Simple PFO or PFO with a non-prominent ASA PFO where a secure device position and effective PFO closure can be achieved when using the 25 mm device size |
| 25 |
| Complex PFO PFO with one or more anatomical characteristics that may complicate the ability to achieve a secure device position and effective PFO closure when using the 25 mm device size |
| 30 or 35 |
| PFO with small anatomy Anatomy not suitable for 25 mm device size secondary to interference with adjacent cardiac structures |
| 18 |
Note: Evaluate the position of the device after deployment, but before detachment. Use echocardiography to ensure that the device does not impinge on the free atrial wall or aortic root. If the device interferes with an adjacent cardiac structure (such as free atrial wall or aortic root), recapture the device and redeploy. If device position remains unsatisfactory, recapture the device and either replace with a smaller device (18 mm or 25 mm) or refer the patient for alternative treatment. The presence of an ASA alone does not necessarily prevent successful PFO closure with a 25 mm device size. In RESPECT1, 180 patients (36%) in the device closure group had an ASA. The 25 mm device size was used in the majority of patients with an ASA (77%) to close the PFO, and at 6-months post-implant, effective closure was achieved in 95% of these patients. There were no cases of device embolization in any patient in the study 1. Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022-32. | ||
References
- Tests performed by and data on file at Abbott.
- Abbott data on file.
- Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017; 377: 1022-32.
Rx Only
AMPLATZER™ TALISMAN™ PFO OCCLUDER
Indications for Use
The AMPLATZER™ PFO Occluder is indicated for percutaneous transcatheter closure of a patent foramen ovale (PFO) to reduce the risk of recurrent ischemic stroke in patients, predominantly between the ages of 18 and 60 years, who have had a cryptogenic stroke due to a presumed paradoxical embolism, as determined by a neurologist and cardiologist following an evaluation to exclude known causes of ischemic stroke.
Contraindications
Patients with intra-cardiac mass, vegetation, tumor or thrombus at the intended site of implant, or documented evidence of venous thrombus in the vessels through which access to the PFO is gained; Patients whose vasculature, through which access to the PFO is gained, is inadequate to accommodate the appropriate sheath size; Patients with anatomy in which the AMPLATZER™ PFO device size required would interfere with other intracardiac or intravascular structures, such as valves or pulmonary veins; Patients with other source of right-to-left shunts, including an atrial septal defect and/or fenestrated septum; Patients with active endocarditis or other untreated infections
Potential Complications and Adverse Events
Potential adverse events that may occur during or after a procedure using this device may include, but are not limited to: Air embolus; Allergic drug reaction; Allergic dye reaction; Allergic metal reaction: Nitinol (nickel, titanium), platinum/iridium, stainless steel (chromium, iron, manganese, molybdenum, nickel); Anesthesia reactions; Apnea; Arrhythmia; Bacterial endocarditis; Bleeding; Brachial plexus injury; Cardiac perforation; Cardiac tamponade; Cardiac thrombus; Chest pain; Device embolization; Device erosion; Deep vein thrombosis; Death; Endocarditis; Esophagus injury; Fever; Headache/migraine; Hypertension/hypotension; Myocardial infarction; Pacemaker placement secondary to PFO device closure; Palpitations; Pericardial effusion; Pericardial tamponade; Pericarditis; Peripheral embolism; Pleural effusion; Pulmonary embolism; Reintervention for residual shunt/device removal; Sepsis; Stroke; Transient ischemic attack; Thrombus; Valvular regurgitation; Vascular access site injury; Vessel perforation
Amplatzer™ Talisman™ Delivery Sheath

Indications for Use
The Amplatzer™ Talisman™ Delivery Sheath is indicated to provide a pathway through which an Amplatzer™ PFO Occluder is introduced for patent foramen ovale closure.
Contraindications
- None known.
Warnings
- This device was sterilized with ethylene oxide and is for single use only. Do not reuse or resterilize this device. Attempts to resterilize this device can cause a malfunction, insufficient sterilization, or harm to the patient.
- Do not use this device if the sterile package is open or damaged. Inspect all components before use. Do not use if the package or items appear to be damaged or defective.
- DO NOT use the Amplatzer™ Talisman™ Delivery Sheath after the Use-by date stated on the package label.
- This device should be used only by physicians who are trained in standard transcatheter techniques. The physician should determine which patients are candidates for procedures that use this device.
- Use a hemostasis valve to impede blood backflow during the implant procedure.
Precautions
- The physician should exercise clinical judgment in situations that involve the use of antithrombotic drugs before, during, and/or after the use of the delivery sheath.
- Use caution and rely on imaging guidance when advancing the sheath and dilator to minimize the risk of cardiovascular injury or interference with previously implanted medical devices. Whenever possible, advance the sheath and dilator over a guidewire.
- Do not attempt to use a guidewire larger than the maximum diameter specified in the Directions for Use.
- Do not use a power injection system to put contrast solution through the sheath.
- Remove the dilator and sheath from the patient slowly to prevent an ingress of air.
Potential Adverse Events
Potential adverse events that may occur during or after a procedure using this device may include, but are not limited to:
- Potential adverse events that may occur during or after a procedure using this delivery sheath may include, but are not limited to: • Allergic reaction/toxic effects due to hypersensitivity to contrast agent, anesthesia, device materials, or drugs used to minimize blood clot formation
- Arrhythmia
- Arteriovenous fistulae
- Bleeding
- Cardiac perforation
- Cardiac tamponade
- Cardiovascular injury
- Death
- Dissection
- Embolism (air, foreign body, and peripheral)
- Hematoma
- Infection
- Myocardial infarction
- Pericardial effusion
- Thromboembolism
- Thrombosis
- Vascular access site injury
MAT-2010366 v3.0