Ablation Technology
Ablation procedures are often long and complex. You need a catheter that offers reliability and comfortable usability, and helps you achieve successful clinical outcomes. We offer innovations in ablation technologies that support your needs and are tailored for differences in your patients’ anatomies and conditions.
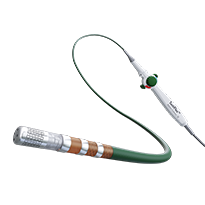
TactiFlex Ablation Catheter, Sensor Enabled
The first and only contact force catheter with a flexible tip, TactiFlex™ Ablation Catheter, Sensor Enabled™ was designed for optimal safety and stability, confident lesion creation and procedural efficiency, and offers full integration with the revolutionary EnSite™ X EP System.1
References
- Nair D et al. Acute Results of a Novel Flexible Tip Radiofrequency Catheter Incorporating Contact Force Sensing. EP Europace. (In press)
Indications, Safety & Warnings
TactiFlex™ Ablation Catheter, Sensor Enabled™
Rx Only. Brief Summary: Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events, and directions for use.
United States: Required Safety Information
Indications: The TactiFlex™ Ablation Catheter, Sensor Enabled™ is indicated for use in cardiac electrophysiological mapping and for the treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation and concomitant atrial flutter, when used in conjunction with a compatible RF generator and three-dimensional mapping system. Contraindications: Do not use for any of the following conditions: recent ventriculotomy or atriotomy heart surgery; prosthetic valves; active systemic infection; use in coronary vasculature; myxoma or intracardiac thrombus, transseptal approach with an interatrial baffle or patch; retrograde trans-aortic approach in patients with aortic valve replacement; patients unable to receive heparin or an acceptable alternative to achieve adequate anticoagulation. Warnings: The temperature data transmitted by the sensor in this catheter is representative of the irrigated electrode only and does not provide tissue temperature data. Application of RF energy on the left atrial posterior wall exceeding 40 W in power, or use of contact force ≥15g, increases the risk of esophageal perforating complications including atrio-esophageal fistula and death. Application of RF energy outside of the power and duration recommendations may increase the likelihood of steam pop occurrence. Patients undergoing septal accessory pathway ablation are at risk for complete AV block which requires the implantation of a permanent pacemaker. Implantable pacemakers and implantable cardioverter/defibrillator (ICDs) may be adversely affected by RF current. The combination of intracoronary placement of the ablation catheter and RF energy application has been associated with myocardial infarction and death. Inspect tubing, connections, and saline irrigation for air bubbles prior to and throughout its use in the procedure. Air or bubbles in the saline irrigation may cause emboli, potential injury, or fatality. Increased contact force may increase the risk for perforation during manipulation of the catheter. Contact force in excess of 20 g may not significantly change the characteristics of lesion formation. Contact force accuracy above 50 g has not been established. Caution should be taken when placing lesions in the proximity of the specialized conduction system. To avoid thromboemboli, intravenous heparin should be used when entering the left heart during ablation. Always maintain a constant saline irrigation flow to prevent coagulation within the lumen of the catheter. When using the catheter with conventional EP lab system (using fluoroscopy to determine catheter tip location) or with a 3D navigational system, careful catheter manipulation must be performed, especially when used in combination with a long sheath, in order to avoid cardiac damage, perforation, or tamponade. Precautions: Always straighten the catheter tip before insertion or withdrawal. If irrigation flow is interrupted, immediately inspect and re flush the catheter outside of the patient. Re-establish irrigation flow prior to placing catheter in the body. Irrigated ablation systems have been shown to create larger lesions than standard radiofrequency ablation catheters. Be careful when ablating near electrically vulnerable, thin walled, or other arterial structures. Potential Adverse Events: Potential adverse events include, but are not limited to, cardiovascular related complications, including hematoma, pericardial effusion and infection. More serious complications are rare, which can include damage to the heart or blood vessels; blood clots (which may lead to stroke); tamponade; severe pulmonary vein stenosis; heart block; myocardial infarction; esophageal fistula, or death.
MAT-2501136 v1.0