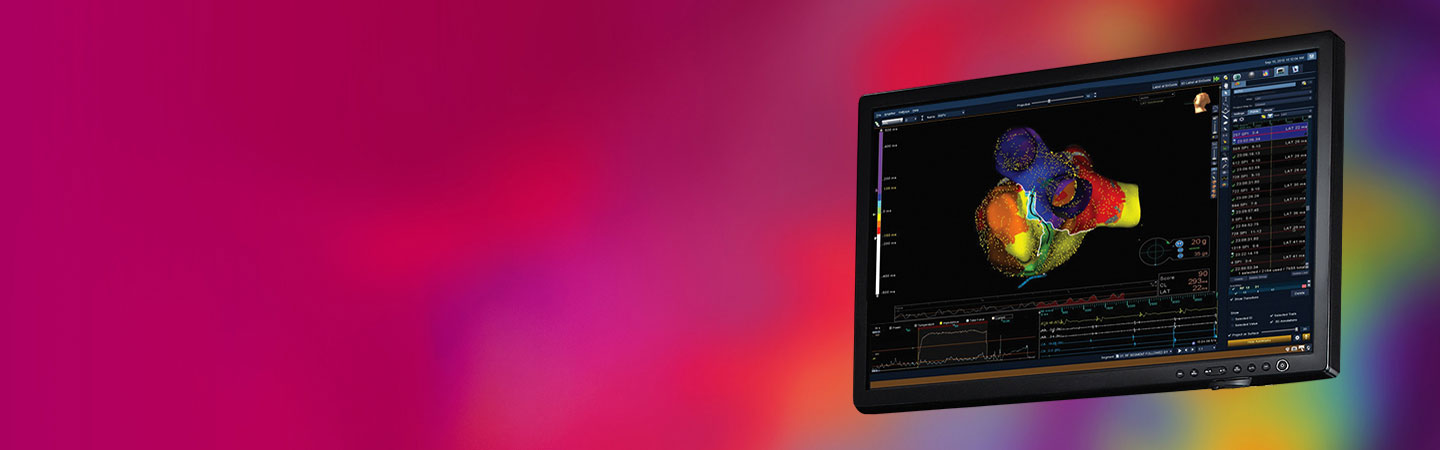
About
The EnSite Precision™ Cardiac Mapping System offers next-generation technology that allows for a high level of automation, flexibility and precision to help you diagnose a wide range of arrhythmias.1,2*
Automated. Flexible. Precise. Transform Procedures with Intuitive Automation1
Advanced mapping is complex. You need precise information quickly to make sound decisions. The EnSite Precision Cardiac Mapping System helps you decrease mapping time using intelligent automation tools.1**
The system:
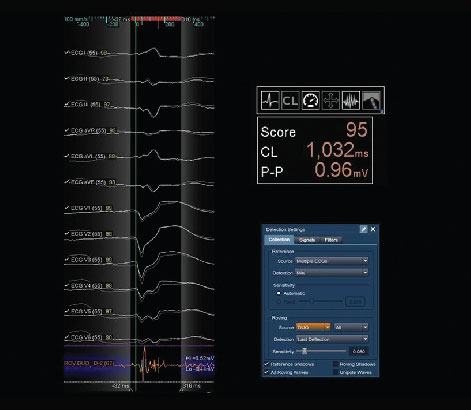
- Enhances VT mapping with automated, advanced morphology matching capability when using the EnSite™ AutoMap module and TurboMap feature.1
- Lets you automatically reject catheter ectopy
- Features new timing detection algorithms including Last Deflection
- Offers two new map types: Fractionation Map and Score Map
Map the Most Complex Cases1
The EnSite Precision Cardiac Mapping System is designed to help you map even the most complex arrhythmia cases through a high level of automation, flexibility and precision.1,2*
EnSite™ AutoMap Module with Automated Morphology Matching Capability
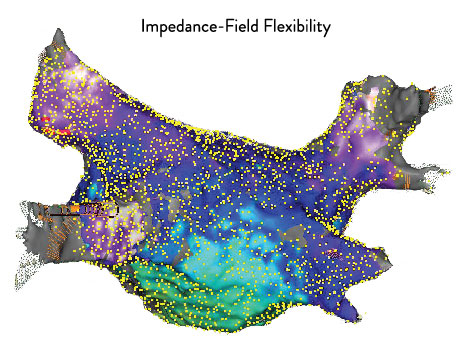
Expand Procedural Options Using Superior Flexibility.1*
The EnSite Precision Cardiac Mapping System is designed to help you customize your procedures to address the circumstances of each case.
- Map any chamber with any catheter.***
- Create faster high-density maps using any catheter.1***
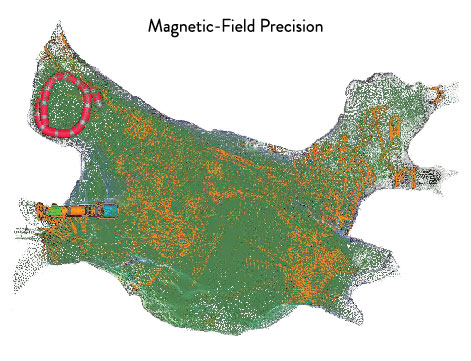
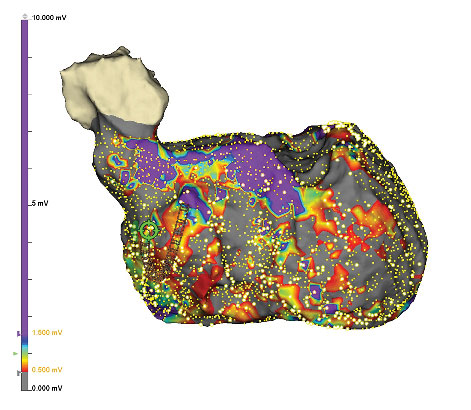
Effectively Manage Patients Through Greater Precision2****
The EnSite Precision Cardiac Mapping System uniquely combines impedance and magnetics, so you can enhance model creation.2
Optimize New and Existing Advanced Technology
Built on an open platform, the EnSite Precision Cardiac Mapping System lets you choose the diagnostic and ablation catheters that best meet the needs of your patients. The EnSite Precision Cardiac Mapping System allows you to:
- Choose contact or noncontact mapping
- Improve model accuracy by reducing the “tenting” effect when creating a model with the ablation catheter, and minimize variation in voltage amplitude during mapping with the EnSite™ contact force module3
- Achieve fast, real-time contact force measurements using the TactiCath™ Ablation Catheter, Sensor Enabled™
- Use EnSite Automap module with OneModel and OneMap in one system, achieving 54% faster, real-time model creation with the OneMap tool for precise anatomic modeling4,5


References
* The open-platform feature of the EnSite Precision™ cardiac mapping system allows for use of almost any catheter for 3D data collection, thus offering superior flexibility as compared to Biosense Webster’s Carto‡ system, which limits 3D data collection to Biosense Webster‡ catheters only.
** Comparison is versus manual mapping with EnSite Precision mapping module or manual lesion marking.
*** In accordance with catheter indication for use.
**** Greater precision based on improvement in accuracy of impedance model with magnetic field scaling applied via robot testing vs. EnSite Velocity v4.0.2.
- Ptaszek LM, Moon B, Rozen G, Mahapatra S, Mansour M. (2018). Novel automated point collection software facilitates rapid, high-density electroanatomic mapping with multiple catheter types. J Cardiovasc Electrophysiol, 29(1), 186-195. https://doi.org/10.1111/jce.13368.
- St. Jude Medical. Data on file, Report 90237452.
- St. Jude Medical. Data on file, Report 90214738.
- Heist, E. K., Danik, S., Chalhoub, F., Koci, F., Barrett, C., Perna, F., … Mansour, M. (2011). Human and animal feasibility study of investigational 3D geometry acquisition software. Heart Rhythm, 8, S241, https://dx.doi.org/10.1016/j.hrthm.2011.03.027
- Abbott. Data on File. Report 90212729.
MAT-2414992 v2.0