Deliverability Redefined. TAVI Reimagined.
Portico with FlexNav TAVI System provides an experience like no other, is a system designed for performance and a clear choice for every TAVI case. The FlexNav delivery system's exceptional design was purposefully built to give you complete, independent control of valve delivery. Portico with FlexNav brings together innovation across every aspect of its design, offering remarkable flexibility and exceptionally smooth tracking.
Learn about the clinical performance of the Portico with FlexNav TAVI System.
Discover Deliverability.
An Experience Like No Other.
- Glide through anatomy
- Secure, stable, predictable valve placement
- Position the valve exactly where you intend
A System Designed for Performance.
- Navigate with ease
- Feel the calm during valve deployment
- Make every procedure your best one yet
A Clear Choice for Every TAVI Case.
- From routine to complex cases
- Through femoral or alternative access approaches
- Discover your new workhorse valve
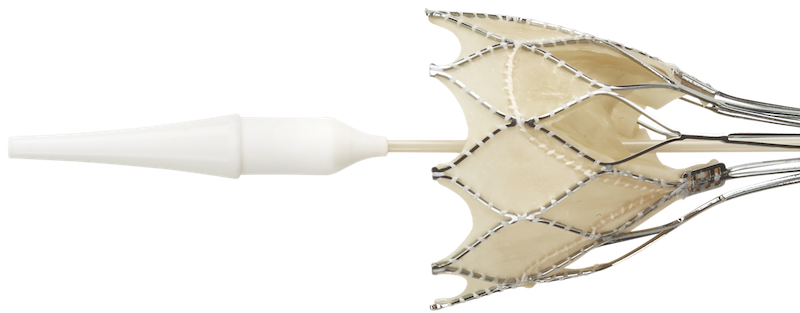
Stable from the Start.
Intra-annular leaflet position in a self-expanding valve provides early valve function and hemodynamic stability throughout the procedure—for calm and controlled deployment, without compromise.
Ordering Information
Portico™ Transcatheter Aortic Valve
| Catalog Number | Valve Size (mm) | Annulus Range (mm)1 | Area Range (mm²)2 | Perimeter Range (mm)2 |
|---|---|---|---|---|
| PRT-23 | 23 | 19-21 | 277–346 | 60–66 |
| PRT-25 | 25 | 21–23 | 338–415 | 66–73 |
| PRT-27 | 27 | 23–25 | 405–491 | 72–79 |
| PRT-29 | 29 | 25–27 | 479–573 | 79–85 |
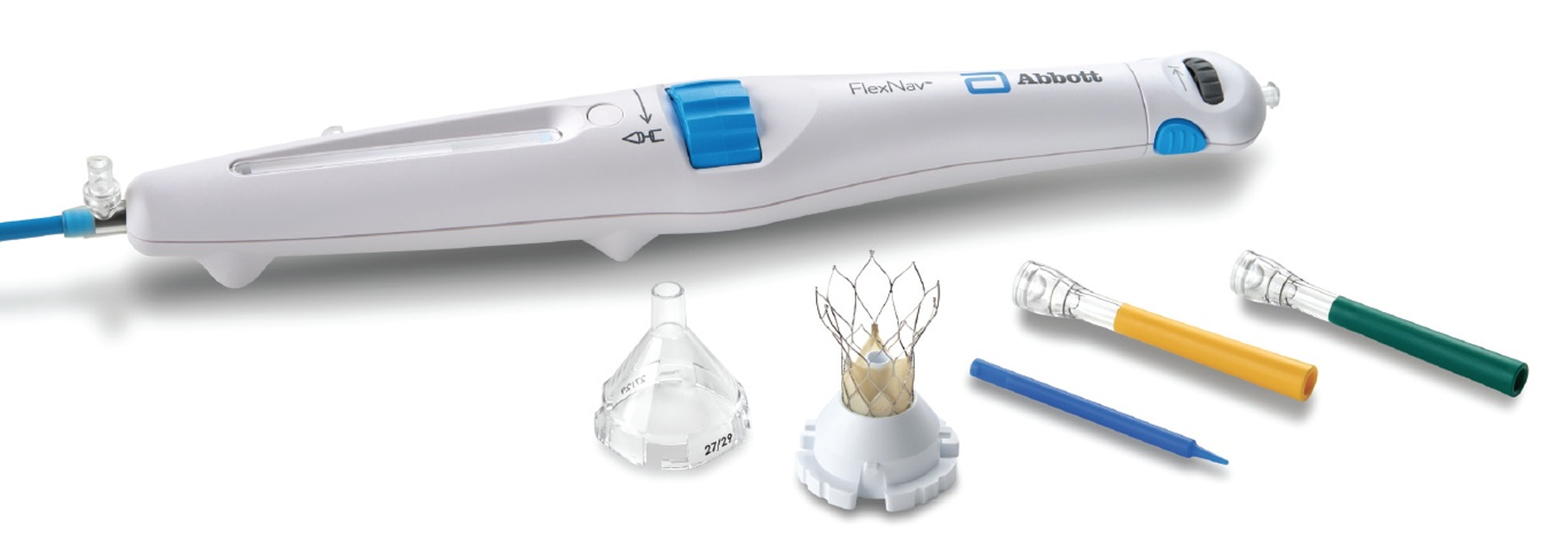
FlexNav™ Delivery System
| Catalog Number | Equivalent Integrated Sheath Diameter (F) | Outer Diameter (mm) | Integrated Sheath Working Length (cm) | Delivery System Length (cm) | Minimum Vessel Diameter Requirement (mm) |
|---|---|---|---|---|---|
| FNAV-DS-SM | 14 | 6.0 | 30 | 107 | ≥ 5.0 |
| FNAV-DS-LG | 15 | 6.3 | 30 | 107 | ≥ 5.5 |
FlexNav™ Loading System
| Catalog Number | |
|---|---|
| FNAV-LS-SM | The FlexNav™ loading system facilitates valve preparation/loading onto the FlexNav delivery system. The loading system includes a loading funnel, loading base, base insert, loading tube, and leaflet tester. |
| FNAV-LS-LG |
MAT-1901243 v2.0
