Femostop™ Gold FCS will be retired outside the United States by the end of 2025. Look to Femostop™ II Plus FCS as your alternative compression assist device!

Fully Integrated Compression Offers Precise, Efficient Hemostasis
This system features an integrated digital manometer and a transparent, inflatable dome that offers precise, hands-free compression of the femoral artery or vein. Compared to manual compression, FemoStop™ Gold has been shown to significantly reduce the incidence of peripheral vascular complications.1
Fully Integrated Femoral Compression System
Offers Precise Placement and Control
- Integrated manometer allows pressure to be adjusted based on patient status.
- The inflatable, transparent dome offers precise pressure for effective hemostasis.
- Adjustable belt designed to fit securely so small patient movements may not cause the device to slip.
Improves Staff Efficiency and Safety
- Hands-free femoral artery or vein compression allows staff to monitor multiple patients at one time.
- Transparent dome ensures puncture site visibility.
- Hands-free compression minimizes staff exposure to blood and reduces neck, arm, and wrist fatigue.
| Component | Function |
|---|---|
| Manometer | Provides user-guided pressure control |
| Pneumatic Pressurized Dome | Translates pressure from manometer to arterial access site |
| Belt | Stabilizes device position and placement |
| Compression Arch | Connects pneumatic pressurized dome, manometer, and belt |
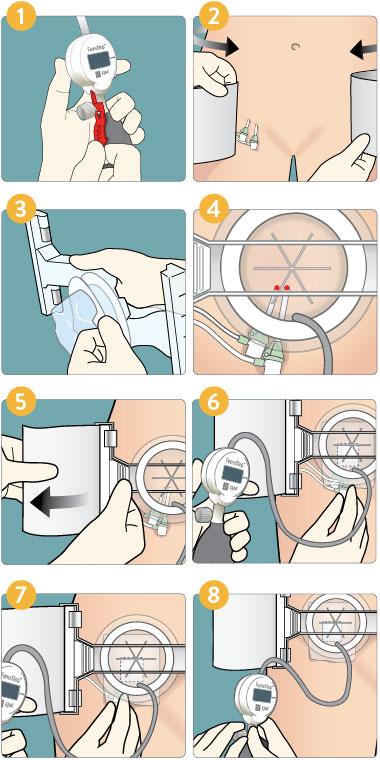
Deployment
- Pull the red tab to activate the pump.
- Position belt in line with puncture site(s), equal on both sides.
- Peel back lid, keeping the dome sterile.
- Center dome over arterial puncture (superior and medial to skin incision). Keep sheath hubs clear of dome rim.
- Attach belt to arch and fit snug. Ensure arch is level and square across the groin area. Adjust by pulling on appropriate corner of belt to make arch perpendicular to femoral compression site.
- Inflate dome to 20-30 mmHg; then remove venous sheath* (if applicable).
- Further inflate to 60-80 mmHg and remove arterial sheath (if applicable). Then quickly increase pressure in dome to be +10-20 mmHg greater than systolic BP or higher as necessary to maintain initial hemostasis.
- After 3 minutes maximum; lower to a maintenance pressure until limb profusion is restored to baseline and hemostasis is maintained. Check pedal pulse periodically to confirm whether or not flow remains in vessel. After appropriate duration as defined by hospital guidelines; lower by 10-20 mmHg every few minutes until you reach zero. Leave in place at low pressure if appropriate. Remove carefully. Dress wound. Discard device.
Ordering Information
| FemoStop™ Gold Femoral Compression System | ||
|---|---|---|
| Part Number | Description | Quantity |
| C11165 | (1) Compression Arch with Pneumatic Dome (1) Integrated Digital Manometer (1) Adjustable Belt (1) Pinch Clamp | 10 per box |
FemoStop™ Gold Femoral Compression System — Instructions for Use (IFU). Refer to the IFU for additional information.
References
- Sridhar K, Fischman D, Goldberg S, et al. Peripheral vascular complications after intracoronary stent placement: prevention by use of a pneumatic vascular compression device. Cathet Cardiovasc Diagn. 1996;39(3):224-229.
MAT-2204318 v2.0
