State-of-the-Art Tip Technology
Chronic total occlusions (CTOs) are prevalent, occurring in 20% of patients undergoing percutaneous coronary intervention (PCI),¹ even though these lesions are seriously undertreated.² Fortunately, successful PCIs for CTOs are steadily increasing—despite the increasing complexity of the lesions attempted—due in part to advances in guide wire technology.²
A penetration wire guide wire—the HI-TORQUE INFILTRAC™ Guide Wire, with its CTO indication—is key to successfully penetrating the CTO cap using the antegrade wire escalation (AWE) technique. The design of this specialized guide wire, including its enhanced technology and performance characteristics, all contribute to CTO procedural efficacy and efficiency.
The HI-TORQUE INFILTRAC™ Guide Wire Designed for CTO Penetration
Micro-Textured Tip: For CTO Cap Engagement
This guide wire’s unique micro-textured tip:
- Provides excellent traction
- Can penetrate even resistant proximal CTO caps
Because the tip is uncoated, it also provides exceptional tactile feedback to the operator.

Tapered Tip: For High Penetration Capabilities
There are two penetration powers available with the HI-TORQUE INFILTRAC guide wire, used with the antegrade wire escalation technique.
| HI‑TORQUE INFILTRAC™ Guide Wire | HI‑TORQUE INFILTRAC™ PLUS Guide Wire | |
|---|---|---|
| Tip Load | 11 g | 14 g |
| Penetration Power | 167 kg/in² | 224 kg/in² |
With either choice of guide wire, the tip is tapered to 0.009” to improve penetration capability. In addition to penetration power, the tapered, pre-shaped micro-J tip is a critical feature enabling CTO PCI success.

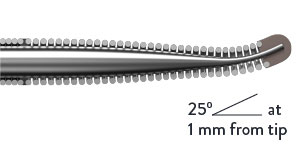
Pre-Shaped Micro-J Tip
The HI-TORQUE INFILTRAC™ Guide Wire’s micro-J tip has a 25° angle extending 1 mm from the tip. This enhances steering to help penetrate CTO lesions.
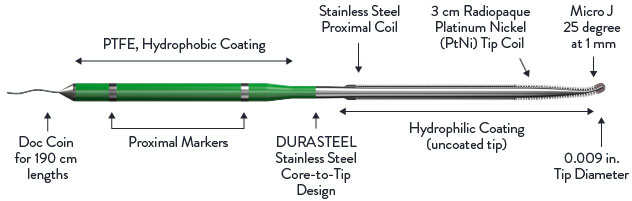
Unique Guide Wire Design
Penetration of challenging CTOs is due to a number of unique design features of the HI-TORQUE INFILTRAC™ Guide Wire:
- Micro-textured tip to engage the CTO cap
- Tapered, pre-shaped tip for high penetration
- Uncoated tip for tactile feedback
Abbott’s proven guide wire design features are also incorporated into the design:
- DURASTEEL stainless steel for needed support
- Hydrophilic coating (excluding the tip) for lubricity and ease of movement within a microcatheter
- Parabolic core grind for excellent torque response
Ordering Information
| Description | non-CE* Part Number | CE Part Number | Tip Load (g) | Penetration Power (kg/in²) | Wire Length (cm) | Tip Diameter (in) | Tip Shape |
|---|---|---|---|---|---|---|---|
| HI-TORQUE INFILTRAC™ Guide Wire | 1030001J | 1030005J | 11 | 167 | 190 | 0.009 | Micro-J |
| 1030002J | 1030006J | 11 | 167 | 300 | 0.009 | Micro-J | |
| HI-TORQUE INFILTRAC™ PLUS Guide Wire | 1030003J | 1030007J | 14 | 224 | 190 | 0.009 | Micro-J |
| 1030004J | 1030008J | 14 | 224 | 300 | 0.009 | Micro-J |
References
* Same as U.S. FDA part numbers applicable to select OUS countries
Data on file at Abbott.
- Van Veelen, A, et al. Incidence and outcomes of chronic total occlusion percutaneous coronary intervention in the Netherlands. Neth Heart J (2021) 29: 4-13.
- Konstantinidis NV, Werner GS, Deftereos S, et al, Euro CTO Club. Temporal Trends in Chronic Total Occlusion Interventions in Europe. Circ Cardiovasc Interv. 2018;11(10):e006229.
MAT-2104932 v2.0
