Tendril STS Pacing Leads*
The clinically proven Tendril STS Pacing Lead has been enhanced with the addition of SurGrip™ suture sleeve technology, providing a new level of secure fixation and advanced lead protection.2
The Tendril STS Pacing Leads with SurGrip suture sleeve technology provides a reliable, safe and no wait, MRI-Ready solution with the performance you can depend on.2 The Tendril STS Pacing Lead is approved for left bundle branch area pacing (LBBAP).*
SurGrip Suture Sleeve Technology
- SurGrip suture sleeve technology provides secure fixation with a uniquely designed channel and novel geomtery1
- Wider spacing and less force results in easier-tie-down, accommodating a range of implant techniques1
- The novel design minimizes possible lead damage due to overtightening, providing enhanced lead protection1

12% increase
in cut-through resistance1
54% increase
in lead crush resistance1
106% increase
in lead slip force resistance (tied to 4 lbs.)1
Reliable
Tendril STS Pacing Lead's reliable performance delivers confidence through actively monitoring long-term data.
>4.6 million
implants worldwide2
16 years
of implants2
97.35%
lead survival at 10 years2
Optim™ Lead Insulation for Long-term Durability
Minimize lead abrasion with Optim lead insulation, a unique hybrid material that:
- Blends the biostability and flexibility of silicone with the durability and lubricity of polyurethane
- Provides abrasion resistance to minimize the risk of lead-on-lead and lead-on-can abrasion in
multi-lead systems3 - Offers flexibility and lubricity for improved handling3
Safe
Safer 6 French (F) design features a thin profile pacing lead designed for flexibility and ease of handling for a safer and easier venous introduction.4
Thin, 6F Profile Design:4
- Improves venous passage and accommodates additional leads within a single vessel
- Reduces the risk of rib or clavicle crush and venous thrombosis
- Affords more options for lead access in a small cephalic vein
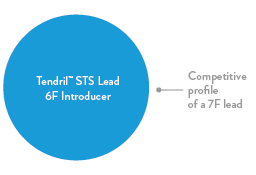
Thin Lead Profile
Thin lead body for safer and easier venous introduction.4 Ease implantation with the small diameter lead, compatible with a 6F introducer.
Soft Silicone Tip4
Soft silicone tip allows for more compliance at the lead-endocardium interface and enhances implant safety.
- Four raised pads on the silicone tip increase the heart tissue and lead contact surface area
- Increase in lead tip surface area of 35% over 6F leads without a soft silicone tip
- Unique soft silicone tip technology that can reduce lead tip pressure by 30%
An increase in lead tip surface
area of 35%4

A tip pressure reduction of 30% (mean)4
No Wait MRI**
Ensures patients can get the care they need, when they need it, with MRI-Ready
portfolio solutions.
Zero days wait time
versus 42 days for competitive systems5-7
1.5T and 3T
both MRI-Ready systems
22.7%
of MRI scans that are urgent or emergent versus 42 days for competitive systems8
Tendril STS Pacing Leads MR Conditional Features***
| Magnet (Tesla) | 1.5T, 3T |
|---|---|
| Scan Type | Full-body |
| Scanner Mode | Normal Operating Mode |
References
*Not approved in all geographies
**No miminum length of time requirement between implant and MRI scan. Stability of pacing capture thresholds is required prior to MRI scan.
***For additional information about specific MR Conditional systems and lead model numbers, including warnings, precautions, adverse conditions to MRI scanning and potential adverse events, please refer to the Abbott MRI-Ready Systems Manual or check our MRI-Ready resources.
- 6Fr Suture Sleeve R&T Data. Doc. 90619024.
- Abbott. Product Performance Report. 2024. 2nd edition. 91086985
- Jenney C, Tan J, Karicherla A, Burke J, Helland J. A new insulation material for cardiac leads with potential for improved performance. Heart Rhythm. 2005;2(5).
- Mechanical and System Product Verification Test Report Tendril STS 1988TC and 2088TC, Report No.50009843/50025428.
- Medtronic Azure‡ MRI Technical Manual, M964377A001 B.
- Boston Scientific ImageReady‡ MR Conditional Pacing System Technical Manual, 36 016 7-003.
- Biotronik ProMRI‡ System Technical Manual, MN062 r20.
- Strom JB, Whelan JB, Shen C, Zheng SQ, Mortele KJ, Kramer DB. Safety and utility of magnetic resonance.
- Mechanical and System Product Verification Test Report Tendril STS 1988TC and 2088TC, Report No.50009843/50025428.
MAT-2201450 v2.0
